Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
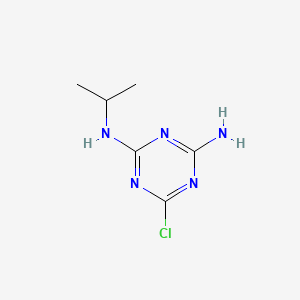

1. 2-chloro-4-amino-6-(isopropylamino)-s-triazine
2. 2-ciat
3. 4-deethylatrazine
1. 6190-65-4
2. Atrazine-desethyl
3. Desethyl Atrazine
4. 4-deethylatrazine
5. Deethylatrazin
6. Desethylatrazine
7. Des-ethyl Atrazine
8. 6-chloro-n2-isopropyl-1,3,5-triazine-2,4-diamine
9. Atrazine Desethyl
10. 2-chloro-4-amino-6-(isopropylamino)-s-triazine
11. 2-chloro-4-isopropylamino-6-amino-s-triazine
12. 6-chloro-2-n-propan-2-yl-1,3,5-triazine-2,4-diamine
13. Ciat
14. Desisopropyl Propazine
15. 6-chloro-n-(1-methylethyl)-1,3,5-triazine-2,4-diamine
16. 2-amino-4-isopropylamino-6-chlorotriazine
17. S-triazine, 2-amino-4-chloro-6-(isopropylamino)-
18. 07pv14bk6x
19. 1,3,5-triazine-2,4-diamine, 6-chloro-n-(1-methylethyl)-
20. 2-chloro-4-amino-6-isopropylamino-s-triazine
21. Chebi:28212
22. S-chloroaminoisopropylaminotriazine
23. 2-amino-4-chloro-6-(isopropylamino)-s-triazine
24. 2-amino-4-isopropylamino-6-chloro-s-triazine
25. 6-chloro-n-isopropyl-1,3,5-triazine-2,4-diamine
26. 6-chloro-n-(propan-2-yl)-1,3,5-triazine-2,4-diamine
27. Ccris 3555
28. Hsdb 2672
29. Unii-07pv14bk6x
30. Deethyatrazine
31. Desethyl-atrazine
32. 6-chloro-n-isopropyl-1,3,5-triazine-2,4-diamine (atrazine-desethyl)
33. G 30033
34. Deethylatratone
35. 2-amino-4-chloro-6-(isopropylamino)-1,3,5-triazine
36. N-deethylatrazine
37. Atrazine M (des-ethyl)
38. Atrazine-desethyl Solution
39. Deethylatrazine, 4-
40. Dsstox_cid_17494
41. Dsstox_rid_79322
42. Dsstox_gsid_37494
43. 2-amino-4-(isopropylamino)-6-chloro-1,3,5-triazine
44. Schembl1425222
45. Chembl3184909
46. Desethyl Atrazine [hsdb]
47. Dtxsid5037494
48. Zinc896284
49. Tox21_301071
50. Atrazine-desethyl, Analytical Standard
51. Akos004119348
52. Sb73302
53. Ncgc00163770-01
54. Ncgc00163770-02
55. Ncgc00163770-03
56. Ncgc00163770-04
57. Ncgc00163770-05
58. Ncgc00254972-01
59. Cas-6190-65-4
60. Db-082361
61. Atrazine-desethyl 100 Microg/ml In Methanol
62. Cs-0454252
63. Ft-0661868
64. Atrazine-desethyl 10 Microg/ml In Acetonitrile
65. Atrazine-desethyl 100 Microg/ml In Cyclohexane
66. C06559
67. Atrazine-desethyl 100 Microg/ml In Acetonitrile
68. G-30033
69. 2-amino-4-chloro-6-isopropylamino-1,3,5-triazine
70. 2-chloro-4-amino-6-isopropylamino-1,3,5-triazine
71. 2-amino-4-chloro-6-isopropylamino-s-triazine
72. 6-amino-2-chloro-4-isopropylamino-s-triazine
73. Atrazine-desethyl, Pestanal(r), Analytical Standard
74. Q22330060
75. 2-isopropylamino-4-amino-6-chloro-1,3,5-triazine
76. 6-chloro-2-n-(propan-2-yl)-1,3,5-triazine-2,4-diamine
77. 6-chloro-n-(1-methylethyl)-1,3,5-triazine-2,4-diamine, 9ci
78. 1,3,5-triazine-2,4-diamine, 6-chloro-n2-(1-methylethyl)-
79. Atrazine-desethyl Solution, 100 Mug/ml In Methanol, Pestanal(r), Analytical Standard
| Molecular Weight | 187.63 g/mol |
|---|---|
| Molecular Formula | C6H10ClN5 |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Exact Mass | 187.0624730 g/mol |
| Monoisotopic Mass | 187.0624730 g/mol |
| Topological Polar Surface Area | 76.7 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 142 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
... The atrazine (ATZ) concentrations in urine samples of the workers collected from an atrazine plant were determined by /a gas chromatograph-electron capture detector/ method /for detecting ATZ and its metabolites (deethylatrazine (DEA), deisopropylatrazine (DIA), deethyldeisopropylatrazine (DEDIA)) in human urine/. The concentration ranges were 0.003 -0.301 mg/L for DEDIA, 0.005 -0.011 mg/L for DEA, 0.006 -0.276 mg/L for DIA, and 0.005 -0.012 mg/L for ATZ.
PMID:18161334 Xu R et al; Se Pu. 25 (5): 758-61 (2007)
... After atrazine ingestion, the urine and carcass of treated animals /contained/ atrazine and its metabolites, indicating that atrazine was absorbed through the GI tract. /Atrazine & metabolites/
USEPA; Health and Environmental Effects Profile for Atrazine p.22 (1984) ECAO-CIN-P098
Deethylatrazine (desethyl atrazine) is a metabolite of atrazine.
PMID:512229 ERICKSON MD ET AL; J AGRIC FOOD CHEM 27 (4): 743-6 (1979)
Three species of Pseudomonas capable of utilizing atrazine as a sole source of carbon were isolated by enrichment from soil with a long history of atrazine application. Atrazine was metabolized via N-dealkylation with preferential formation of deisopropylatrazine over deethylatrazine. Two of the species were able to carry out the following incubation in glucose-supplemented mineral salts medium.
Behki RM and Khan Su; J Agric Food Chem 34 (4): 748-9 (1986)
Deethylatrazine is primarily a mammalian metabolite but can be produced in plants and bacteria. Dealkylation of the ethyl group from the 4 position of the triazine ring yields deethylatrazine.
USEPA; Revised Toxicology Chapter; Interim Reregistration Eligibility Decision (IRED) for Atrazine (1912-24-9). Available from, as of Nov 20, 2003: https://www.epa.gov/oppsrrd1/reregistration/atrazine/
Manganese enhanced atrazine transformation by the fungus Pleurotus pulmonarius when added to a liquid culture medium at concentration of up to 300 uM. Both N-dealkylated and propylhydroxylated metabolites accumulated in the culture medium, with the former accumulating to a greater extent than did the latter. Lipid peroxidation, oxygenase and peroxidase activities, and the cytochrome P-450 concentration increased. In addition, an increase in the spectral interactions between atrazine and components in the cell extract was observed. Antioxidants, mainly nordihydroguaiaretic acid, which inhibits lipoxygenase, peroxidase, and P-450 activities, and piperonyl butoxide, which inhibits P-450 activity, inhibited atrazine transformation by the mycelium. It is suggested that the stimulation of oxidative activity by manganese might be responsible for increasing the biotransformation of atrazine and for nonspecific transformations of other xenobiotic compounds.
PMID:8967773 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC168164 Masaphy S et al; Applied and Environmental Microbiology 62 (1): 3587-3593 (1996)
ABOUT THIS PAGE
17
PharmaCompass offers a list of Deethylatrazine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Deethylatrazine manufacturer or Deethylatrazine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Deethylatrazine manufacturer or Deethylatrazine supplier.
PharmaCompass also assists you with knowing the Deethylatrazine API Price utilized in the formulation of products. Deethylatrazine API Price is not always fixed or binding as the Deethylatrazine Price is obtained through a variety of data sources. The Deethylatrazine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Deethylatrazine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Deethylatrazine, including repackagers and relabelers. The FDA regulates Deethylatrazine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Deethylatrazine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Deethylatrazine supplier is an individual or a company that provides Deethylatrazine active pharmaceutical ingredient (API) or Deethylatrazine finished formulations upon request. The Deethylatrazine suppliers may include Deethylatrazine API manufacturers, exporters, distributors and traders.
Deethylatrazine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Deethylatrazine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Deethylatrazine GMP manufacturer or Deethylatrazine GMP API supplier for your needs.
A Deethylatrazine CoA (Certificate of Analysis) is a formal document that attests to Deethylatrazine's compliance with Deethylatrazine specifications and serves as a tool for batch-level quality control.
Deethylatrazine CoA mostly includes findings from lab analyses of a specific batch. For each Deethylatrazine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Deethylatrazine may be tested according to a variety of international standards, such as European Pharmacopoeia (Deethylatrazine EP), Deethylatrazine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Deethylatrazine USP).
