
Synopsis
Synopsis
0
JDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
South Africa

DRUG PRODUCT COMPOSITIONS
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
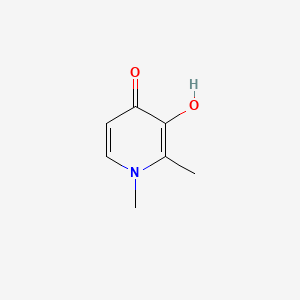

1. 1,2 Dimethyl 3 Hydroxy 4 Pyridinone
2. 1,2 Dimethyl 3 Hydroxypyrid 4 One
3. 1,2 Dimethyl 3 Hydroxypyridin 4 One
4. 1,2-dimethyl-3-hydroxy-4-pyridinone
5. 1,2-dimethyl-3-hydroxypyrid-4-one
6. 1,2-dimethyl-3-hydroxypyridin-4-one
7. 3 Hydroxy 1,2 Dimethyl 4 Pyridinone
8. 3-hydroxy-1,2-dimethyl-4-pyridinone
9. Dmohpo
10. Ferriprox
11. Hdmpp
1. 30652-11-0
2. 3-hydroxy-1,2-dimethyl-4(1h)-pyridone
3. Ferriprox
4. 3-hydroxy-1,2-dimethylpyridin-4(1h)-one
5. Cp20
6. 1,2-dimethyl-3-hydroxy-4-pyridone
7. 1,2-dimethyl-3-hydroxypyrid-4-one
8. Apo-66
9. 1,2-dimethyl-3-hydroxypyridine-4-one
10. 4(1h)-pyridinone, 3-hydroxy-1,2-dimethyl-
11. Apo-066
12. Dn-180-01-af
13. 3-hydroxy-1,2-dimethylpyridin-4-one
14. Deferipron
15. Mfcd00134497
16. Deferiprone (inn)
17. Pl-1
18. L1
19. Chebi:68554
20. 1,2-dimethyl-3-hydroxy-4(1h)-pyridinone
21. Cp-20
22. Dn-18001af
23. Nsc-758880
24. 2bty8kh53l
25. Mls000069481
26. Hk-1
27. Pl1
28. 4(1h)-pyridinone, 1,2-dimethyl-3-hydroxy-
29. Smr000059136
30. Deferiprone [inn]
31. L-1
32. Cp20 (chelating Agent)
33. Ferriprox (tn)
34. Unii-2bty8kh53l
35. Brn 1447108
36. Deferidone
37. Deferiprona
38. Deferum
39. Deferiprone [usan:inn:ban]
40. Ccris 8318
41. 1,2-dimethyl-3-hydroxy-4-pyridinone
42. 3-hydroxy-1,2-dimethyl-4-pyridinone
43. L-1[chelating Agent]
44. Opera_id_366
45. 3-hydroxy-1,2-dimethyl-4(1h)-pyridinone
46. Deferiprone [mi]
47. 4(1h)-pyridone, 3-hydroxy-1,2-dimethyl-
48. Deferiprone (usan/inn)
49. Deferiprone [usan]
50. Deferiprone [vandf]
51. Deferiprone [mart.]
52. Dsstox_cid_20666
53. Dsstox_rid_79529
54. Dsstox_gsid_40666
55. Schembl94474
56. Deferiprone [who-dd]
57. Mls000758227
58. Mls001424029
59. Mls006011689
60. Chembl70927
61. Deferiprone [ema Epar]
62. Crmd-001
63. Gtpl7456
64. Zinc6226
65. Dtxsid6040666
66. Hsdb 8335
67. Tzxkocqbrnjulo-uhfffaoysa-
68. Deferiprone [orange Book]
69. Ex-a972
70. Cp020
71. Deferiprone [ep Monograph]
72. Hms2051c05
73. Hms2232e24
74. Hms3264p17
75. Hms3393c05
76. Hms3873l13
77. Pharmakon1600-01504512
78. Bcp09606
79. Hy-b0568
80. 3-hydroxy-1,2-dimethylpyrid-4-one
81. Tox21_112429
82. Bbl036482
83. Bdbm50525976
84. Cgp-37391
85. Nsc758880
86. S4067
87. Stl559018
88. 1,2-dimetyl-3-hydroxy-4-pyridinone
89. Akos015902286
90. Ccg-100760
91. Cs-5240
92. Db08826
93. Ds-5493
94. Nc00010
95. Nsc 758880
96. 3-hydroxy-1,2-dimethyl-4- Pyridinone
97. 3-hydroxy-1,2-dimethyl-pyridin-4-one
98. Ncgc00090548-01
99. Ncgc00090548-02
100. Ncgc00090548-04
101. Ncgc00090548-05
102. Ac-33026
103. Bp-12415
104. Sy052576
105. Sbi-0207041.p001
106. Cas-30652-11-0
107. Db-047826
108. 1,2-dimethyl-3-hydroxy-4-pyridone, 99+%
109. Ft-0606424
110. H0944
111. D07416
112. 3-hydroxy-1,2-dimethyl-4(1h)-pyridone, 98%
113. Ab00572605_11
114. Ab00572605_12
115. A820470
116. Q749664
117. Sr-01000721891
118. Deferiprone 1,2-dimethyl-3-hydroxy-4-pyridone
119. Sr-01000721891-4
| Molecular Weight | 139.15 g/mol |
|---|---|
| Molecular Formula | C7H9NO2 |
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 139.063328530 g/mol |
| Monoisotopic Mass | 139.063328530 g/mol |
| Topological Polar Surface Area | 40.5 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 228 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Ferriprox |
| PubMed Health | Deferiprone (By mouth) |
| Drug Classes | Heavy Metal Chelator |
| Drug Label | Ferriprox (deferiprone) tablets contain 500mg deferiprone (3-hydroxy-1,2-dimethylpyridin-4-one), a synthetic, orally active, iron-chelating agent. Deferiprone has the following structural formula:Deferiprone is a white to pinkish-white crystallin... |
| Active Ingredient | Deferiprone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Apopharma |
| 2 of 2 | |
|---|---|
| Drug Name | Ferriprox |
| PubMed Health | Deferiprone (By mouth) |
| Drug Classes | Heavy Metal Chelator |
| Drug Label | Ferriprox (deferiprone) tablets contain 500mg deferiprone (3-hydroxy-1,2-dimethylpyridin-4-one), a synthetic, orally active, iron-chelating agent. Deferiprone has the following structural formula:Deferiprone is a white to pinkish-white crystallin... |
| Active Ingredient | Deferiprone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Apopharma |
Iron Chelating Agents
National Library of Medicine's Medical Subject Headings. Deferiprone. Online file (MeSH, 2016). Available from, as of June 24, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Deferiprone is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of July 6, 2016: https://clinicaltrials.gov/search/intervention=DEFERIPRONE
Ferriprox (deferiprone) is indicated for the treatment of patients with transfusional iron overload due to thalassemia syndromes when current chelation therapy is inadequate. /Included in US product label/
NIH; DailyMed. Current Medication Information for Ferriprox (Deferiprone) Tablet, Film-coated (Updated: February 2015). Available from, as of July 14, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc8cbc3d-026c-0db5-42e8-8e93d374dd23
/EXPL THER/ Friedreich ataxia is an inherited disorder characterized by degeneration of the peripheral and central nervous system and hypertrophic cardiomyopathy. Homozygous mutations in the frataxine (FXN) gene reduce expression of frataxin and cause accumulation of iron in the mitochondria. Deferiprone, an oral iron chelator, has been shown effective in cell and animal models of Friedreich ataxia. The results of a 6-month randomized, double blind placebo-controlled study suggested that deferiprone 20 mg/kg/day may reduce disease progression. The authors present their experience of 5 Friedreich ataxia patients treated with deferiprone (20 mg/kg/day), in addition to idebenone treatment, followed over a period of 10-24 months, under off-label authorization. The patients were monitored for laboratory parameters, cardiac assessment, neurological evaluations, and quality of life. The authors conclude that combined therapy of a low dose of deferiprone with idebenone is relatively safe, might improve neurological function, and seems to improve heart hypertrophy, warranting further studies.
PMID:27029487 Elincx-Benizri S et al; J Child Neurol 31 (8): 1036-40 (2016)
/EXPL THER/ Growing body of evidence suggests that Parkinson's disease (PD) is associated with oxidative damage via iron accumulation in the substantia nigra (SN). Low ceruloplasmin (CP)-ferroxidase activity has been identified in the SN and the cerebrospinal fluid (CSF) of patients with PD. The iron chelator, deferiprone, reduces the abnormally high levels of iron in the SN. In order to determine CP's involvement in iron accumulation in SN and PD progression, we aim to compare the ability of iron chelation treatment to reducing both SN iron levels and motor handicap in PD patients according to the level of ceruloplasmin activity. We used a moderate chelation protocol with deferiprone (DFP) based on a, 6-month delayed-start paradigm, randomized placebo controlled clinical trial in 40 PD patients. CP-ferroxidase activity was determined in blood and CSF together with the D544E gene polymorphism (rs701753). Iron levels were determined by R2* MRI sequence and the motor handicap by the UPDRS motor score. After 6 to 12 months of DFP treatment, greater reductions in SN iron levels and UPDRS motor scores were obtained in patients with higher serum and CSF levels of CP-ferroxidase activity. After 6 months of DFP treatment, the AT genotype group displayed greater reduction of iron level in the SN with greater CSF and serum levels of CP activity than the AA genotype group. Although most of the DFP-treated patients displayed clinical and radiological improvements, those with the lower CP activity appeared to respond better to iron chelation. Larger RCTs are now needed to establish whether pharmacological modulation of CP activity could be an innovative neuroprotective strategy in PD.
PMID:25943368 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4429376 Grolez G et al; BMC Neurol 15:74 (2015)
/BOXED WARNING/ WARNING: AGRANULOCYTOSIS/NEUTROPENIA: Ferriprox can cause agranulocytosis that can lead to serious infections and death. Neutropenia may precede the development of agranulocytosis. Measure the absolute neutrophil count (ANC) before starting Ferriprox therapy and monitor the ANC weekly on therapy. Interrupt Ferriprox therapy if neutropenia develops. Interrupt Ferriprox if infection develops, and monitor the ANC more frequently. Advise patients taking Ferriprox to report immediately any symptoms indicative of infection.
NIH; DailyMed. Current Medication Information for Ferriprox (Deferiprone) Tablet, Film-coated (Updated: February 2015). Available from, as of July 14, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc8cbc3d-026c-0db5-42e8-8e93d374dd23
Based on evidence of genotoxicity and developmental toxicity in animal studies, Ferriprox can cause fetal harm when administered to a pregnant woman. ... If Ferriprox is used during pregnancy or if the patient becomes pregnant while taking Ferriprox, the patient should be apprised of the potential hazard to the fetus.
NIH; DailyMed. Current Medication Information for Ferriprox (Deferiprone) Tablet, Film-coated (Updated: February 2015). Available from, as of July 14, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc8cbc3d-026c-0db5-42e8-8e93d374dd23
It is not known whether deferiprone is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for adverse reactions in nursing infants from Ferriprox, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
NIH; DailyMed. Current Medication Information for Ferriprox (Deferiprone) Tablet, Film-coated (Updated: February 2015). Available from, as of July 14, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc8cbc3d-026c-0db5-42e8-8e93d374dd23
Safety and effectiveness in elderly individuals have not been established. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
NIH; DailyMed. Current Medication Information for Ferriprox (Deferiprone) Tablet, Film-coated (Updated: February 2015). Available from, as of July 14, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc8cbc3d-026c-0db5-42e8-8e93d374dd23
For more Drug Warnings (Complete) data for Deferiprone (13 total), please visit the HSDB record page.
Deferiprone is indicated in thalassemia syndromes when first line chelation agents are not adequate to treat transfusional iron overload.
FDA Label
Ferriprox monotherapy is indicated for the treatment of iron overload in patients with thalassaemia major when current chelation therapy is contraindicated or inadequate.
Ferriprox in combination with another chelator is indicated in patients with thalassaemia major when monotherapy with any iron chelator is ineffective, or when prevention or treatment of life-threatening consequences of iron overload (mainly cardiac overload) justifies rapid or intensive correction.
Deferiprone Lipomed monotherapy is indicated for the treatment of iron overload in patients with thalassaemia major when current chelation therapy is contraindicated or inadequate.
Deferiprone Lipomed in combination with another chelator is indicated in patients with thalassaemia major when monotherapy with any iron chelator is ineffective, or when prevention or treatment of life-threatening consequences of iron overload justifies rapid or intensive correction.
Treatment of chronic iron overload
Iron Chelating Agents
Organic chemicals that form two or more coordination links with an iron ion. Once coordination has occurred, the complex formed is called a chelate. The iron-binding porphyrin group of hemoglobin is an example of a metal chelate found in biological systems. (See all compounds classified as Iron Chelating Agents.)
V03AC02
V03AC02
V03AC02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
V - Various
V03 - All other therapeutic products
V03A - All other therapeutic products
V03AC - Iron chelating agents
V03AC02 - Deferiprone
Absorption
Deferiprone is absorbed in the upper gastrointestinal tract. Absorption is rapid with maximum plasma concentrations occurring after 1 hour in the fasted state and after 2 hours in the fed state.
Route of Elimination
Within 5-6 hours of administration, more than 90% of deferiprone is eliminated from the plasma. 75 to 90% of deferiprone is excreted in the urine as the metabolite.
Volume of Distribution
In healthy patients, the volume of distribution is 1L/kg, and in thalassemia patients, the volume of distribution is 1.6L/kg.
In healthy subjects, the mean maximum concentration (Cmax) of deferiprone in serum was 20 ug/mL, and the mean total area under the concentration-time curve (AUC) was 53 ug*hr/mL following oral administration of a 1,500 mg dose of Ferriprox tablets in the fasting state. Dose proportionality over the labeled dosage range of 25 to 33 mg/kg three times per day (75 to 99 mg/kg per day) has not been studied. The elimination half life of deferiprone was 1.9 hours. The accumulation of deferiprone and its glucuronide metabolite at the highest approved dosage level of 33 mg/kg three times per day has not been studied. The volume of distribution of deferiprone is 1.6 L/kg in thalassemia patients, and approximately 1 L/kg in healthy subjects. The plasma protein binding of deferiprone in humans is less than 10%.
NIH; DailyMed. Current Medication Information for Ferriprox (Deferiprone) Tablet, Film-coated (Updated: February 2015). Available from, as of July 14, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc8cbc3d-026c-0db5-42e8-8e93d374dd23
Deferiprone is rapidly absorbed from the upper part of the gastrointestinal tract, appearing in the blood within 5 to 10 minutes of oral administration. Peak serum concentrations occur approximately 1 hour after a single dose in fasted healthy subjects and patients, and up to 2 hours after a single dose in the fed state. Administration with food decreased the Cmax of deferiprone by 38% and the AUC by 10%. While a food effect cannot be ruled out, the magnitude of the exposure change does not warrant dose adjustment.
NIH; DailyMed. Current Medication Information for Ferriprox (Deferiprone) Tablet, Film-coated (Updated: February 2015). Available from, as of July 14, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc8cbc3d-026c-0db5-42e8-8e93d374dd23
More than 90% of deferiprone is eliminated from plasma within 5 to 6 hours of ingestion. Following oral administration, 75% to 90% is recovered in the urine in the first 24 hours, primarily as metabolite.
NIH; DailyMed. Current Medication Information for Ferriprox (Deferiprone) Tablet, Film-coated (Updated: February 2015). Available from, as of July 14, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc8cbc3d-026c-0db5-42e8-8e93d374dd23
/MILK/ It is not known whether deferiprone is excreted in human milk.
NIH; DailyMed. Current Medication Information for Ferriprox (Deferiprone) Tablet, Film-coated (Updated: February 2015). Available from, as of July 14, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc8cbc3d-026c-0db5-42e8-8e93d374dd23
For more Absorption, Distribution and Excretion (Complete) data for Deferiprone (8 total), please visit the HSDB record page.
Deferiprone is mainly metabolized by UGT1A6 to the 3-O-glucuronide metabolite. This metabolite cannot chelate iron.
In humans, the majority of the deferiprone is metabolized, primarily by UGT1A6. The contribution of extrahepatic (e.g., renal) UGT1A6 is unknown. The major metabolite of deferiprone is the 3-O-glucuronide, which lacks iron binding capability. Peak serum concentration of the glucuronide occurs 2 to 4 hours after administration of deferiprone in fasting subjects.
NIH; DailyMed. Current Medication Information for Ferriprox (Deferiprone) Tablet, Film-coated (Updated: February 2015). Available from, as of July 14, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc8cbc3d-026c-0db5-42e8-8e93d374dd23
The half-life is 1.9 hours.
The pharmacokinetics of deferiprone in children was assessed in 7 patients with thalassemia and iron overload aged 11 to 18 years (mean age= 15 + or - 2.7 years; median=16 years). These patients were on long term therapy with deferiprone and were thus considered to be at steady state. ... Serum levels of deferiprone were maximal approximately 2 hours after dosing and declined with a half-life of 1.8 hours; levels of deferiprone glucuronide peaked at approximately 3 hours and fell with a half-life of 2.0 hours. ...
Health Canada; Product Monograph for Ferriprox (Deferiprone) Tablets (500 mg and 1000 mg) Deferiprone Oral Solution (100 mg/mL). Drug Identification Number (DIN): 02436531 p.17 (Date of Preparation: January 22, 2015). Available from, as of July 14, 2016: https://webprod5.hc-sc.gc.ca/dpd-bdpp/start-debuter.do?lang=eng
In healthy subjects ... following oral administration of a 1,500 mg dose of Ferriprox tablets in the fasting state ... the elimination half life ... was 1.9 hours.
NIH; DailyMed. Current Medication Information for Ferriprox (Deferiprone) Tablet, Film-coated (Updated: February 2015). Available from, as of July 14, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc8cbc3d-026c-0db5-42e8-8e93d374dd23
Deferiprone is an iron chelator that binds to ferric ions (iron III) and forms a 3:1 (deferiprone:iron) stable complex and is then eliminated in the urine. Deferiprone is more selective for iron in which other metals such as zinc, copper, and aluminum have a lower affinity for deferiprone.
Deferiprone is a chelating agent with an affinity for ferric ion (iron III). Deferiprone binds with ferric ions to form neutral 3:1 (deferiprone:iron) complexes that are stable over a wide range of pH values. Deferiprone has a lower binding affinity for other metals such as copper, aluminum and zinc than for iron.
NIH; DailyMed. Current Medication Information for Ferriprox (Deferiprone) Tablet, Film-coated (Updated: February 2015). Available from, as of July 14, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc8cbc3d-026c-0db5-42e8-8e93d374dd23

127.8
51 - 200
25.9k
3.3M
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|---|---|---|
| INDONESIA | 3,367.35 | 149.8 | 11 - 50 |
| CANADA | 12,994.34 | 106.0 | 11 - 50 |
| BANGLADESH | 1,195.00 | 127.9 | 11 - 50 |
| PAKISTAN | 700.23 | 93.9 | <10 |
| VIETNAM, DEMOCRATIC REP. OF | 1,850.00 | 140.0 | <10 |
| VIETNAM | 1,526.00 | 118.6 | <10 |
| IRAQ | 570.00 | 139.4 | <10 |
| GREECE | 1,804.54 | 183.2 | <10 |
| NETHERLANDS | 868.03 | 185.5 | <10 |
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
56
PharmaCompass offers a list of Deferiprone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Deferiprone manufacturer or Deferiprone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Deferiprone manufacturer or Deferiprone supplier.
PharmaCompass also assists you with knowing the Deferiprone API Price utilized in the formulation of products. Deferiprone API Price is not always fixed or binding as the Deferiprone Price is obtained through a variety of data sources. The Deferiprone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Deferiprone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Deferiprone, including repackagers and relabelers. The FDA regulates Deferiprone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Deferiprone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Deferiprone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Deferiprone supplier is an individual or a company that provides Deferiprone active pharmaceutical ingredient (API) or Deferiprone finished formulations upon request. The Deferiprone suppliers may include Deferiprone API manufacturers, exporters, distributors and traders.
click here to find a list of Deferiprone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Deferiprone DMF (Drug Master File) is a document detailing the whole manufacturing process of Deferiprone active pharmaceutical ingredient (API) in detail. Different forms of Deferiprone DMFs exist exist since differing nations have different regulations, such as Deferiprone USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Deferiprone DMF submitted to regulatory agencies in the US is known as a USDMF. Deferiprone USDMF includes data on Deferiprone's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Deferiprone USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Deferiprone suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Deferiprone Drug Master File in Korea (Deferiprone KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Deferiprone. The MFDS reviews the Deferiprone KDMF as part of the drug registration process and uses the information provided in the Deferiprone KDMF to evaluate the safety and efficacy of the drug.
After submitting a Deferiprone KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Deferiprone API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Deferiprone suppliers with KDMF on PharmaCompass.
A Deferiprone CEP of the European Pharmacopoeia monograph is often referred to as a Deferiprone Certificate of Suitability (COS). The purpose of a Deferiprone CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Deferiprone EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Deferiprone to their clients by showing that a Deferiprone CEP has been issued for it. The manufacturer submits a Deferiprone CEP (COS) as part of the market authorization procedure, and it takes on the role of a Deferiprone CEP holder for the record. Additionally, the data presented in the Deferiprone CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Deferiprone DMF.
A Deferiprone CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Deferiprone CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Deferiprone suppliers with CEP (COS) on PharmaCompass.
A Deferiprone written confirmation (Deferiprone WC) is an official document issued by a regulatory agency to a Deferiprone manufacturer, verifying that the manufacturing facility of a Deferiprone active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Deferiprone APIs or Deferiprone finished pharmaceutical products to another nation, regulatory agencies frequently require a Deferiprone WC (written confirmation) as part of the regulatory process.
click here to find a list of Deferiprone suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Deferiprone as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Deferiprone API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Deferiprone as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Deferiprone and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Deferiprone NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Deferiprone suppliers with NDC on PharmaCompass.
Deferiprone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Deferiprone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Deferiprone GMP manufacturer or Deferiprone GMP API supplier for your needs.
A Deferiprone CoA (Certificate of Analysis) is a formal document that attests to Deferiprone's compliance with Deferiprone specifications and serves as a tool for batch-level quality control.
Deferiprone CoA mostly includes findings from lab analyses of a specific batch. For each Deferiprone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Deferiprone may be tested according to a variety of international standards, such as European Pharmacopoeia (Deferiprone EP), Deferiprone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Deferiprone USP).
