
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
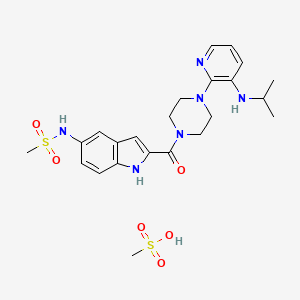

1. Delavirdine
2. Mesylate, Delavirdine
3. Rescriptor
4. U 90152
5. U 90152s
6. U-90152
7. U-90152s
8. U90152
9. U90152s
1. 147221-93-0
2. Delavirdine (mesylate)
3. Rescriptor
4. Delavirdine Mesilate
5. U-90152s
6. Delavirdine Methanesulfonate
7. Delavirdine Mesylate [usan]
8. Delavirdine Monomethanesulfonate
9. N-(2-(4-(3-(isopropylamino)pyridin-2-yl)piperazine-1-carbonyl)-1h-indol-5-yl)methanesulfonamide Methanesulfonate
10. Chebi:4379
11. 1-(3-(isopropylamino)-2-pyridyl)-4-((5-methanesulfonamidoindol-2-yl)carbonyl)piperazine Monomethanesulfonate
12. 421105krqe
13. Methanesulfonamide, N-[2-[[4-[3-[(1-methylethyl)amino]-2-pyridinyl]-1-piperazinyl]carbonyl]-1h-indol-5-yl]-, Methanesulfonate (1:1)
14. Delavirdine Mesilate (jan)
15. Delavirdine Mesylate (usan)
16. U 90152 (mesylate);bhap-u 90152 (mesylate)
17. Delavirdine Mesilate [jan]
18. Piperazine, 1-(3-((1-methylethyl)amino)-2-pyridinyl)-4-((5-((methylsulfonyl)amino)-1h-indol-2-yl)carbonyl)-, Monomethanesulfonate
19. Unii-421105krqe
20. Sr-05000001471
21. Hsdb 7162
22. Rescriptor (tn)
23. Drg-0166
24. U 90152e
25. U 90152s
26. U 90152t
27. Chembl929
28. U 90152 (mesylate)
29. Schembl40522
30. Bhap-u 90152 (mesylate)
31. Dtxsid701017136
32. Delavirdine Mesylate [hsdb]
33. Bcp09460
34. Delavirdine Mesylate [vandf]
35. Delavirdine Mesilate [mart.]
36. Hy-10571a
37. S6452
38. Delavirdine Mesylate [who-dd]
39. Akos024458004
40. At12830
41. Cs-1661
42. Delavirdine Mesylate, >=98% (hplc)
43. Delavirdine Methanesulfonate [mi]
44. Bs-15144
45. Delavirdine Mesylate [orange Book]
46. U-90152t
47. Ft-0603116
48. C08087
49. D00895
50. J-008332
51. Sr-05000001471-2
52. Q27068164
53. 1-(3-(isopropylamino)-2-pyridyl)-4-((5-methanesulphonamidoindol-2-yl)carbonyl)piperazine Monomethanesulphonate
54. 1-[3-[(1-methylethyl)amino]-2-pyridinyl]-4-[[5-[(methylsulfonyl)amino]-1h-indol-2-yl]carbonyl]-piperazine Monomethanesulfonate
55. Methanesulfonic Acid;n-[2-[4-[3-(propan-2-ylamino)pyridin-2-yl]piperazine-1-carbonyl]-1h-indol-5-yl]methanesulfonamide
56. N-(2-(4-(3-(isopropylamino)pyridin-2-yl)piperazine-1-carbonyl)-1h-indol-5-yl)methanesulfonamidemethanesulfonate
57. N-[2-({4-[3-(propan-2-ylamino)pyridin-2-yl]piperazin-1-yl}carbonyl)-1h-indol-5-yl]methanesulfonamide Methanesulfonate
58. N-{2-[4-(3-isopropylamino-pyridin-2-yl)-piperazine-1-carbonyl]-1h-indol-5-yl}-methanesulfonamide; Compound With Methanesulfonic Acid
59. Piperazine, 1-(3-((1-methylethyl)amino)-2-pyridinyl)-4-((5-((methylsulphonyl)amino)-1h-indol-2-yl)carbonyl)-, Monomethanesulphonate
| Molecular Weight | 552.7 g/mol |
|---|---|
| Molecular Formula | C23H32N6O6S2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 6 |
| Exact Mass | 552.18247511 g/mol |
| Monoisotopic Mass | 552.18247511 g/mol |
| Topological Polar Surface Area | 182 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 842 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Rescriptor |
| PubMed Health | Delavirdine (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | RESCRIPTOR Tablets contain delavirdine mesylate, a synthetic non-nucleoside reverse transcriptase inhibitor of the human immunodeficiency virus type 1 (HIV-1). The chemical name of delavirdine mesylate is piperazine, 1-[3-[(1-methyl-ethyl)amino]-2-py... |
| Active Ingredient | Delavirdine mesylate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 100mg |
| Market Status | Prescription |
| Company | Viiv Hlthcare |
| 2 of 2 | |
|---|---|
| Drug Name | Rescriptor |
| PubMed Health | Delavirdine (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | RESCRIPTOR Tablets contain delavirdine mesylate, a synthetic non-nucleoside reverse transcriptase inhibitor of the human immunodeficiency virus type 1 (HIV-1). The chemical name of delavirdine mesylate is piperazine, 1-[3-[(1-methyl-ethyl)amino]-2-py... |
| Active Ingredient | Delavirdine mesylate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 100mg |
| Market Status | Prescription |
| Company | Viiv Hlthcare |
Delavirdine is indicated in the treatment of HIV-1 infection in combination with other appropriate antiretroviral therapy. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1042
Plasma lipid and lipoprotein levels were measured at baseline and after 8 weeks of highly active antiretroviral therapy among patients receiving delavirdine with or without a protease inhibitor (PI). In patients receiving nucleoside reverse transcriptase inhibitors (NRTI) plus delavirdine, there was a statistically significant increase in cholesterol and HDL levels, whereas those receiving NRTI plus a PI had no significant change in their HDL levels. When delavirdine was combined with a PI, there was a more dramatic increase in both cholesterol and HDL concentrations.
PMID:12218397 Roberts AD et al; AIDS 16 (13): 1829-30 (2002)
Rash is the most frequently reported adverse effect of delavirdine. In 2 clinical studies..., rash was reported in 32-35% of adults who received the usual dosage of delavirdine in conjunction with nucleoside reverse transcriptase inhibitors compared with 16-21% of adults who received the nucleoside reverse transcriptase inhibitors alone. In these studies, grade 1 rash (erythema, pruritus), grade 2 rash (diffuse maculopapular rash, dry desquamation), or grade 3 rash (vesiculation, moist desquamation, ulceration) occurred in about 17, 14, and 4%, respectively, of patients receiving delavirdine in conjunction with nucleoside reverse transcriptase inhibitors compared with 12, 6, and 0%, respectively, of patients receiving the nucleoside reverse transcriptase inhibitors alone.There were no cases of grade 4 rash (erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, necrosis requiring surgery, exfoliative dermatitis) in either patient group. About 3-4% of patients receiving delavirdine in conjunction with nucleoside reverse transcriptase inhibitors discontinued treatment because of rash compared with 0-1% of patients who received the nucleoside reverse transcriptase inhibitors alone.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 633
Delavirdine-associated rash occurs mainly on the upper body and proximal arms with decreasing intensity of the lesions on the neck and face and progressively less on the rest of the trunk and limbs. Rash usually is evident within the first few weeks following initiation of delavirdine therapy; occurrence of rash after 1 month of therapy is uncommon. Because there is no evidence that the incidence of rash is decreased by initiating delavirdine therapy using a low dose and then titrating dosage, dosage titration is not recommended.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 633
Severe and life-threatening skin reactions, including Stevens-Johnson syndrome and erythema multiforme, have been reported rarely in patients receiving delavirdine; 2 cases of Stevens-Johnson syndrome were reported during postmarketing surveillance. These severe reactions resolved after the drug was discontinued..
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 633
For more Drug Warnings (Complete) data for DELAVIRDINE MESYLATE (26 total), please visit the HSDB record page.
Anti-HIV Agents
Agents used to treat AIDS and/or stop the spread of the HIV infection. These do not include drugs used to treat symptoms or opportunistic infections associated with AIDS. (See all compounds classified as Anti-HIV Agents.)
Reverse Transcriptase Inhibitors
Inhibitors of reverse transcriptase (RNA-DIRECTED DNA POLYMERASE), an enzyme that synthesizes DNA on an RNA template. (See all compounds classified as Reverse Transcriptase Inhibitors.)
Elimination: Fecal: 44% following multiple doses of 330 mg three times a day in healthy volunteers. Renal: 51%, following multiple doses of 330 mg three times a day in healthy volunteers. Less than 5% of the dose is recovered unchanged in urine.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1043
Delavirdine is distributed predominantly into blood plasma.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1042
Delavirdine is well absorbed, especially at pH less than 2.0.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1362
Delavirdine mesylate is rapidly absorbed following oral administration, and peak plasma concentrations of the drug are attained approximately 1 hour after the dose. Following oral administration of 400 mg of delavirdine mesylate 3 times daily in HIV-infected adults, mean steady-state peak plasma concentrations of the drug are 15.98 ug/ml (range: 0.91-45.66 ug/ml), mean trough plasma concentrations are 6.85 ug/ml (range: 0.05-20.55 ug/ml), and mean AUC is 82.19 ughour/ml.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 638
For more Absorption, Distribution and Excretion (Complete) data for DELAVIRDINE MESYLATE (8 total), please visit the HSDB record page.
Delavirdine binds extensively to plasma proteins and primarily is metabolized by CYP3A4. The major metabolic pathway results in N-dealkylation. There is considerable intersubject variability in plasma delavirdine concentrations related to differences in CYP3A activity. The CSF-to-plasma ratio is 0.02.
Rom, W.N. (ed.). Environmental and Occupational Medicine. 2nd ed. Boston, MA: Little, Brown and Company, 1992., p. 1362
The metabolism of delavirdine in the mouse was extensive and involved amide bond cleavage, N-desalkylation, hydroxylation at the C-6' position of the pyridine ring, and pyridine ring-cleavage as determined by MS and/or 1H and 13C NMR spectroscopies. N-desalkylation and amide bond cleavage were the primary metabolic pathways at low drug doses and, as the biotransformation of delavirdine to desalkyl delavirdine reached saturation or inhibition, amide bond cleavage became the predominant pathway at higher doses and after multiple doses.
PMID:9224777 Chang M et al; Drug Metab Dispos 25 (7): 828-39 (1997)
The apparent plasma half-life of delavirdine increases with dose. The mean plasma half-life of delavirdine is 5.8 hours (range: 2-11 hours) in adults receiving a dosage of 400 mg 3 times daily.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 638
Elimination from plasma: Mean, 5.8 hours (range, 2 to 11 hours) following treatment with 400 mg three times a day. The apparent half-life increases with dose.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1042
After entering the cell, delavirdine binds to a hydrophobic pocket in the p66 subunit of reverse transcriptase. This causes a conformational change to a stable, inactive form of the enzyme. The delavirdine-reverse transcriptase complex is stabilized by hydrogen bonds at residue Lys-103 and strong hydrophobic interactions with residue Pro-236. Much higher concentrations of delavirdine are required to inhibit cellular polymerase than reverse transcriptase.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1362
While the complete mechanism of antiviral activity of delavirdine has not been fully elucidated, the drug inhibits replication of human immunodeficiency virus type 1 (HIV-1) by interfering with viral RNA- and DNA-directed polymerase activities of reverse transcriptase. HIV reverse transcriptase is essential for viral replication, and its activities occur in the host cell cytoplasm after the viral particle penetrates the cell membrane and releases the viral core, but before nuclear entry and chromosomal integration of proviral DNA. The enzyme is multifunctional, with 3 principal activities (ie., RNA-directed DNA polymerase, RNase H, and DNA-directed DNA polymerase functions). Using viral RNA as a template, reverse transcriptase forms a minus strand of viral DNA, creating a double-stranded RNA:DNA hybrid (i.e., RNA-directed DNA polymerase function). The RNase H function of reverse transcriptase facilitates copying of viral RNA by degrading the RNA component of the RNA:DNA hybrid after the RNA is copied, leaving a single minus strand of viral DNA. Using the newly formed minus strand of viral DNA as a template, reverse transcriptase forms the plus strand of viral DNA, converting single-stranded viral DNA to the double-stranded proviral DNA form (i.e, DNA-directed DNA polymerase function). BHAP derivatives, including delavirdine, inhibit the polymerase functions, but not the RNase H function, of reverse transcriptase. The drugs bind directly to heterodimeric HIV-1 reverse transcriptase and exert a virustatic effect by acting as a specific, noncompetitive HIV-1 reverse transcriptase inhibitor.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 637
Nonnucleoside reverse transcriptase inhibitors affect reverse transcriptase at a different site than nucleoside reverse transcriptase inhibitors (e.g., abacavir, didanosine, lamivudine, stavudine, zalcitabine, zidovudine), and the drugs have different mechanisms of action. Unlike currently available nonnucleoside reverse transcriptase inhibitors, dideoxynucleoside antiretroviral agents require intracellular conversion to triphosphate metabolites, which then compete with naturally occurring deoxynucleoside triphosphates for incorporation into viral DNA by reverse transcriptase and cause premature viral DNA chain termination by preventing further 5 to 3 phosphodiester linkages.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 637
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Patents & EXCLUSIVITIES
ABOUT THIS PAGE
29
PharmaCompass offers a list of Delavirdine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Delavirdine manufacturer or Delavirdine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Delavirdine manufacturer or Delavirdine supplier.
PharmaCompass also assists you with knowing the Delavirdine API Price utilized in the formulation of products. Delavirdine API Price is not always fixed or binding as the Delavirdine Price is obtained through a variety of data sources. The Delavirdine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A DELAVIRDINE MESYLATE manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of DELAVIRDINE MESYLATE, including repackagers and relabelers. The FDA regulates DELAVIRDINE MESYLATE manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. DELAVIRDINE MESYLATE API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A DELAVIRDINE MESYLATE supplier is an individual or a company that provides DELAVIRDINE MESYLATE active pharmaceutical ingredient (API) or DELAVIRDINE MESYLATE finished formulations upon request. The DELAVIRDINE MESYLATE suppliers may include DELAVIRDINE MESYLATE API manufacturers, exporters, distributors and traders.
DELAVIRDINE MESYLATE Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of DELAVIRDINE MESYLATE GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right DELAVIRDINE MESYLATE GMP manufacturer or DELAVIRDINE MESYLATE GMP API supplier for your needs.
A DELAVIRDINE MESYLATE CoA (Certificate of Analysis) is a formal document that attests to DELAVIRDINE MESYLATE's compliance with DELAVIRDINE MESYLATE specifications and serves as a tool for batch-level quality control.
DELAVIRDINE MESYLATE CoA mostly includes findings from lab analyses of a specific batch. For each DELAVIRDINE MESYLATE CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
DELAVIRDINE MESYLATE may be tested according to a variety of international standards, such as European Pharmacopoeia (DELAVIRDINE MESYLATE EP), DELAVIRDINE MESYLATE JP (Japanese Pharmacopeia) and the US Pharmacopoeia (DELAVIRDINE MESYLATE USP).
