
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
NDC API
0
VMF
0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
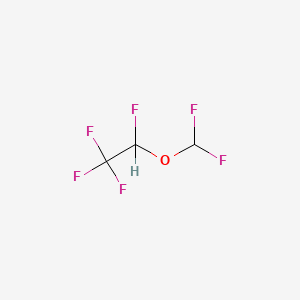

1. 1,2,2,2-tetrafluoroethyl Difluoromethyl Ether
2. I 653
3. I-653
4. I653
5. Suprane
1. 57041-67-5
2. Suprane
3. 2-(difluoromethoxy)-1,1,1,2-tetrafluoroethane
4. Desflurano [inn-spanish]
5. Difluoromethyl 1,2,2,2-tetrafluoroethyl Ether
6. Desfluranum [inn-latin]
7. I-653
8. 1,2,2,2-tetrafluoroethyl Difluoromethyl Ether
9. (+-)-2-difluoromethyl 1,2,2,2-tetrafluoroethyl Ether
10. Crs35bz94q
11. Chebi:4445
12. 1,1,1,2-tetrafluoro-2-(difluoromethoxy)ethane
13. Desfluranum
14. Desflurano
15. Ethane, 2-(difluoromethoxy)-1,1,1,2-tetrafluoro-, (+-)-
16. (+/-)-2-difluoromethyl 1,2,2,2-tetrafluoroethyl Ether
17. I 653
18. Suprane (tn)
19. Unii-crs35bz94q
20. Desflurane (jan/usp/inn)
21. Desfluran-o
22. Hsdb 8058
23. Desflurane [usan:usp:inn:ban]
24. Ethane, 2-(difluoromethoxy)-1,1,1,2-tetrafluoro-
25. Chf2ochfcf3
26. Desflurane [mi]
27. Desflurane [inn]
28. Desflurane [jan]
29. Desflurane [usan]
30. Desflurane [vandf]
31. Desflurane [mart.]
32. Desflurane [usp-rs]
33. Desflurane [who-dd]
34. Schembl62917
35. Ethane,2-(difluoromethoxy)-1,1,1,2-tetrafluoro-
36. Gtpl7156
37. Chembl1200733
38. Desflurane [orange Book]
39. Desflurane [ep Monograph]
40. Dtxsid80866606
41. Desflurane [usp Monograph]
42. Mfcd00236716
43. Akos006228397
44. Db01189
45. Db-053001
46. Difluoromethyl1,2,2,2-tetrafluoroethylether
47. Ft-0624525
48. C07519
49. D00546
50. Q419383
51. Ethane, 2-(difluoromethoxy)-1,1,1,2-tetrafluoro-, (+/-)-
52. 1351959-69-7
| Molecular Weight | 168.04 g/mol |
|---|---|
| Molecular Formula | C3H2F6O |
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 2 |
| Exact Mass | 168.00098366 g/mol |
| Monoisotopic Mass | 168.00098366 g/mol |
| Topological Polar Surface Area | 9.2 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 97.7 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Suprane |
| PubMed Health | Desflurane (By breathing) |
| Drug Classes | Volatile Liquid |
| Drug Label | SUPRANE (desflurane, USP), a nonflammable liquid administered via vaporizer, is a general inhalation anesthetic. It is ()1,2,2,2-tetrafluoroethyl difluoromethyl ether:Some physical constants are:Molecular weight168.04Specific gravity (at 20C/4C... |
| Active Ingredient | Desflurane |
| Dosage Form | Liquid |
| Route | Inhalation |
| Strength | 99.9% |
| Market Status | Prescription |
| Company | Baxter Hlthcare |
| 2 of 2 | |
|---|---|
| Drug Name | Suprane |
| PubMed Health | Desflurane (By breathing) |
| Drug Classes | Volatile Liquid |
| Drug Label | SUPRANE (desflurane, USP), a nonflammable liquid administered via vaporizer, is a general inhalation anesthetic. It is ()1,2,2,2-tetrafluoroethyl difluoromethyl ether:Some physical constants are:Molecular weight168.04Specific gravity (at 20C/4C... |
| Active Ingredient | Desflurane |
| Dosage Form | Liquid |
| Route | Inhalation |
| Strength | 99.9% |
| Market Status | Prescription |
| Company | Baxter Hlthcare |
SUPRANE (desflurane, USP) is indicated as an inhalation agent for induction and/or maintenance of anesthesia for inpatient and outpatient surgery in adults. SUPRANE (desflurane, USP) is not recommended for induction of anesthesia in pediatric patients because of a high incidence of moderate to severe upper airway adverse events (see WARNINGS). After induction of anesthesia with agents other than SUPRANE, and tracheal intubation, SUPRANE is indicated for maintenance of anesthesia in infants and children. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Suprane (desflurane) liquid (April 2011). Available from, as of July 20, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=bf4d8d29-852d-4281-b732-4af0eca019e1
Anesthetics, Inhalation
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009) https://www.nlm.nih.gov/cgi/mesh/2012/MB_cgi?mode=&term=desflurane
SUPRANE (desflurane, USP) may produce a dose-dependent increase in cerebrospinal fluid pressure (CSFP) when administered to patients with intracranial space occupying lesions. Desflurane should be administered at 0.8 MAC or less, and in conjunction with a barbiturate induction and hyperventilation (hypocapnia) until cerebral decompression in patients with known or suspected increases in CSFP. Appropriate attention must be paid to maintain cerebral perfusion pressure.
US Natl Inst Health; DailyMed. Current Medication Information for Suprane (desflurane) liquid (April 2011). Available from, as of July 20, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=bf4d8d29-852d-4281-b732-4af0eca019e1
The concentrations of desflurane in milk are probably of no clinical importance 24 hours after anesthesia. Because of rapid washout, desflurane concentrations in milk are predicted to be below those found with other volatile potent anesthetics.
US Natl Inst Health; DailyMed. Current Medication Information for Suprane (desflurane) liquid (April 2011). Available from, as of July 20, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=bf4d8d29-852d-4281-b732-4af0eca019e1
SUPRANE (desflurane, USP) should be administered only by persons trained in the administration of general anesthesia, using a vaporizer specifically designed and designated for use with desflurane. Facilities for maintenance of a patent airway, artificial ventilation, oxygen enrichment, and circulatory resuscitation must be immediately available. Hypotension and respiratory depression increase as anesthesia is deepened.
US Natl Inst Health; DailyMed. Current Medication Information for Suprane (desflurane) liquid (April 2011). Available from, as of July 20, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=bf4d8d29-852d-4281-b732-4af0eca019e1
For more Drug Warnings (Complete) data for Desflurane (22 total), please visit the HSDB record page.
Desflurane is indicated for the induction and maintenance of anesthesia in adults, as well as the maintenance of anesthesia in pediatric patients.
FDA Label
Desflurane is a general inhalation anesthetic. It has a short duration of action as it is rapidly cleared. Patients should be counselled regarding the risks of malignant hyperthermia, perioperative hyperkalemia, respiratory adverse reactions in pediatric patients, QTc prolongation, hepatobiliary disorders, pediatric neurotoxicity, and postoperative agitation in children.
Anesthetics, Inhalation
Gases or volatile liquids that vary in the rate at which they induce anesthesia; potency; the degree of circulation, respiratory, or neuromuscular depression they produce; and analgesic effects. Inhalation anesthetics have advantages over intravenous agents in that the depth of anesthesia can be changed rapidly by altering the inhaled concentration. Because of their rapid elimination, any postoperative respiratory depression is of relatively short duration. (From AMA Drug Evaluations Annual, 1994, p173) (See all compounds classified as Anesthetics, Inhalation.)
N01AB07
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N01 - Anesthetics
N01A - Anesthetics, general
N01AB - Halogenated hydrocarbons
N01AB07 - Desflurane
Absorption
Data regarding the Cmax, Tmax, and AUC of desflurane are not readily available.
Route of Elimination
Initially, desflurane is rapidly eliminated from the lungs. A small amount of the metabolite trifluoroacetic acid is eliminated in the urine and only 0.02% of an inhaled dose is recovered as urinary metabolites.
Volume of Distribution
Desflurane has a median volume of distribution of 612 mL/kg.
Clearance
A 26 g dose of desflurane is 90% eliminated from the brain after 33 hours. The metabolite trifluoroacetic acid has a urinary clearance rate of 0.169 0.107 mol/L.
The concentrations of desflurane in milk are probably of no clinical importance 24 hours after anesthesia.
US Natl Inst Health; DailyMed. Current Medication Information for Suprane (desflurane) liquid (April 2011). Available from, as of July 20, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=bf4d8d29-852d-4281-b732-4af0eca019e1
Due to the volatile nature of desflurane in plasma samples, the washin-washout profile of desflurane was used as a surrogate of plasma pharmacokinetics. Eight healthy male volunteers first breathed 70% N2O/30% O2 for 30 minutes and then a mixture of SUPRANE (desflurane, USP) 2.0%, isoflurane 0.4%, and halothane 0.2% for another 30 minutes. During this time, inspired and endtidal concentrations (FI and FA) were measured. The FA/FI (washin) value at 30 minutes for desflurane was 0.91, compared to 1.00 for N2O, 0.74 for isoflurane, and 0.58 for halothane. The washin rates for halothane and isoflurane were similar to literature values. The washin was faster for desflurane than for isoflurane and halothane at all time points. The FA/FAO (washout) value at 5 minutes was 0.12 for desflurane, 0.22 for isoflurane, and 0.25 for halothane. The washout for SUPRANE was more rapid than that for isoflurane and halothane at all elimination time points. By 5 days, the FA/FAO for desflurane is 1/20th of that for halothane or isoflurane.
US Natl Inst Health; DailyMed. Current Medication Information for Suprane (desflurane) liquid (April 2011). Available from, as of July 20, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=bf4d8d29-852d-4281-b732-4af0eca019e1
Desflurane is minimally defluorinated by CYP2E1, to the extent that serum fluoride levels do not increase above baseline levels.
Renal and hepatic toxicity of the fluorinated ether volatile anesthetics is caused by biotransformation to toxic metabolites. Metabolism also contributes significantly to the elimination pharmacokinetics of some volatile agents. Although innumerable studies have explored anesthetic metabolism in animals, there is little information on human volatile anesthetic metabolism with respect to comparative rates or the identity of the enzymes responsible for defluorination. The first purpose of this investigation was to compare the metabolism of the fluorinated ether anesthetics by human liver microsomes. The second purpose was to test the hypothesis that cytochrome P450 2E1 is the specific P450 isoform responsible for volatile anesthetic defluorination in humans. Microsomes were prepared from human livers. Anesthetic metabolism in microsomal incubations was measured by fluoride production. The strategy for evaluating the role of P450 2E1 in anesthetic defluorination involved three approaches: for a series of 12 human livers, correlation of microsomal defluorination rate with microsomal P450 2E1 content (measured by Western blot analysis), correlation of defluorination rate with microsomal P450 2E1 catalytic activity using marker substrates (para-nitrophenol hydroxylation and chlorzoxazone 6-hydroxylation), and chemical inhibition by P450 isoform-selective inhibitors. The rank order of anesthetic metabolism, assessed by fluoride production at saturating substrate concentrations, was methoxyflurane > sevoflurane > enflurane > isoflurane > desflurane > 0. There was a significant linear correlation of sevoflurane and methoxyflurane defluorination with antigenic P450 2E1 content (r = 0.98 and r = 0.72, respectively), but not with either P450 1A2 or P450 3A3/4. Comparison of anesthetic defluorination with either para-nitrophenol or chlorzoxazone hydroxylation showed a significant correlation for sevoflurane (r = 0.93, r = 0.95) and methoxyflurane (r = 0.78, r = 0.66). Sevoflurane defluorination was also highly correlated with that of enflurane (r = 0.93), which is known to be metabolized by human P450 2E1. Diethyldithiocarbamate, a selective inhibitor of P450 2E1, produced a concentration-dependent inhibition of sevoflurane, methoxyflurane, and isoflurane defluorination. No other isoform-selective inhibitor diminished the defluorination of sevoflurane, whereas methoxyflurane defluorination was inhibited by the selective P450 inhibitors furafylline (P450 1A2), sulfaphenazole (P450 2C9/10), and quinidine (P450 2D6) but to a much lesser extent than by diethyldithiocarbamate. These results demonstrate that cytochrome P450 2E1 is the principal, if not sole human liver microsomal enzyme catalyzing the defluorination of sevoflurane. P450 2E1 is the principal, but not exclusive enzyme responsible for the metabolism of methoxyflurane, which also appears to be catalyzed by P450s 1A2, 2C9/10, and 2D6. The data also suggest that P450 2E1 is responsible for a significant fraction of isoflurane metabolism. Identification of P450 2E1 as the major anesthetic metabolizing enzyme in humans provides a mechanistic understanding of clinical fluorinated ether anesthetic metabolism and toxicity.
PMID:8214760 Kharasch ED, Thummel KE; Anesthesiology 79 (4): 795-807 (1993)
SUPRANE (desflurane, USP) is a volatile liquid inhalation anesthetic minimally biotransformed in the liver in humans.
US Natl Inst Health; DailyMed. Current Medication Information for Suprane (desflurane) liquid (April 2011). Available from, as of July 20, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=bf4d8d29-852d-4281-b732-4af0eca019e1
Biotransformation and hepatotoxicity of desflurane were evaluated in the guinea pig liver slice culture system. Liver slices (250-300 microns) were prepared from 600-650-g male Hartley guinea pigs. The slices were incubated in sealed vials in a Krebs-Henseleit buffer at 37 degrees C under 95% O2. Desflurane was vaporized to produce media concentrations of 0.7-2.3 mM. After incubation (3-24 hr) viability of the slices was determined (K+ content; protein synthesis secretion) along with the biotransformation of desflurane (F-). Isoflurane (2.3 mM) was included in the studies for comparative purposes. Although desflurane caused a mild concentration-related reduction in slice K+ content (1.1-2.2 mM; 20%-40% of control), the effects were less than those produced by 2.3 mM isoflurane (50% of control). High concentrations of desflurane decreased protein synthesis at the first 9 hr of incubation, and isoflurane decreased protein synthesis throughout the incubation period. Neither anesthetic affected protein secretion. The biotransformation of desflurane was minimal with threefold less F- produced from desflurane than isoflurane.
PMID:2035863 Ghantous HN et al; Anesth Analg 72 (6): 796-800 (1991)
The metabolism of desflurane has been assessed both in animals and humans by measuring the appearance of fluoride metabolites (fluoride ion, nonvolatile organic fluoride, trifluoroacetic acid) in blood and urine. Desflurane administered to rats (either pretreated or not pretreated with phenobarbital or ethanol) for 3.2 MAC-hours and to swine for 5.5 MAC-hours produced fluoride ion levels in blood that were almost indistinguishable from values measured in control animals. In contrast, a significant 17% increase in plasma fluoride ion concentration in swine was detected 4 hr after exposure to desflurane. In human studies, desflurane administered to patients (3.1 MAC-hours) and volunteers (7.35 MAC-hours) resulted in postanesthesia serum fluoride in concentrations that did not differ from background fluoride ion concentrations. Similarly, postanesthetic urinary excretion of fluoride ion and organic fluoride in volunteers was comparable to preanesthetic excretion rates. Small but statistically significant levels of trifluoroacetic acid were found in both serum and urine from volunteers after exposure to desflurane. Peak serum concentrations averaging 0.38 +/- 0.17 uM trifluoroacetic acid (mean +/- SD) and peak urinary excretion rates averaging 0.169 +/- 0.107 umol/hr were detected in volunteers 24 hr after desflurane exposure. Although these increases in trifluoroacetic acid after exposure to desflurane were statistically significant, they are approximately 10-fold less than levels seen after exposure to isoflurane. Desflurane strongly resists biodegradation, and only a small amount is metabolized in animals and humans.
PMID:1524235 Koblin DD; Anesth Analg 75 (4 Suppl): S10-6 (1992)
Desflurane has a terminal elimination half life of 8.16 3.15 minutes.
The mechanism of inhalational anesthetics is still not fully understood. They can block excitatory ion channels and increase the activity of inhibitory ion channels. The most notable agonism is at the GABAA channel. Desflurane is also an agonist of glycine receptors, antagonist of glutamate receptors, inducer of potassium voltage gated channels, and inhibits both NADH-ubiquinone oxioreductase chain 1 and calcium transporting ATPases. An older school of thought is the unitary theory of general anesthetic action, suggesting that desflurane affects the lipid bilayer of cells. Studies of other halogenated inhalational anesthetics have shown that the lipid bilayer spreads out more thinly as the anesthetic incorporates into the bilayer. However, the anesthetic does not bind to lipid heads or acyl chains of hydrocarbons in the bilayer. The effect of incorporating into the lipid bilayer is not well described. By incorporating into the lipid bilayer, anesthetics may introduce disorder in the lipids, leading to some indirect effect on ion channels. However, this theory remains controversial.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code : AN
Brand Name : SUPRANE
Dosage Form : LIQUID;INHALATION
Dosage Strength : 100%
Approval Date : 1992-09-18
Application Number : 20118
RX/OTC/DISCN : RX
RLD : Yes
TE Code : AN

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AN
Brand Name : DESFLURANE
Dosage Form : LIQUID;INHALATION
Dosage Strength : 100%
Approval Date : 2018-02-26
Application Number : 208234
RX/OTC/DISCN : RX
RLD : No
TE Code : AN

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : SUPRANE
Dosage Form : LIQUID
Dosage Strength : 100%/V/V
Packaging : 240ML
Approval Date :
Application Number : 2227428
Regulatory Info : Prescription
Registration Country : Canada

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
60
PharmaCompass offers a list of Desflurane API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Desflurane manufacturer or Desflurane supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Desflurane manufacturer or Desflurane supplier.
PharmaCompass also assists you with knowing the Desflurane API Price utilized in the formulation of products. Desflurane API Price is not always fixed or binding as the Desflurane Price is obtained through a variety of data sources. The Desflurane Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Desflurane manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Desflurane, including repackagers and relabelers. The FDA regulates Desflurane manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Desflurane API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Desflurane manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Desflurane supplier is an individual or a company that provides Desflurane active pharmaceutical ingredient (API) or Desflurane finished formulations upon request. The Desflurane suppliers may include Desflurane API manufacturers, exporters, distributors and traders.
click here to find a list of Desflurane suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Desflurane DMF (Drug Master File) is a document detailing the whole manufacturing process of Desflurane active pharmaceutical ingredient (API) in detail. Different forms of Desflurane DMFs exist exist since differing nations have different regulations, such as Desflurane USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Desflurane DMF submitted to regulatory agencies in the US is known as a USDMF. Desflurane USDMF includes data on Desflurane's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Desflurane USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Desflurane suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Desflurane Drug Master File in Korea (Desflurane KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Desflurane. The MFDS reviews the Desflurane KDMF as part of the drug registration process and uses the information provided in the Desflurane KDMF to evaluate the safety and efficacy of the drug.
After submitting a Desflurane KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Desflurane API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Desflurane suppliers with KDMF on PharmaCompass.
Desflurane Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Desflurane GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Desflurane GMP manufacturer or Desflurane GMP API supplier for your needs.
A Desflurane CoA (Certificate of Analysis) is a formal document that attests to Desflurane's compliance with Desflurane specifications and serves as a tool for batch-level quality control.
Desflurane CoA mostly includes findings from lab analyses of a specific batch. For each Desflurane CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Desflurane may be tested according to a variety of international standards, such as European Pharmacopoeia (Desflurane EP), Desflurane JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Desflurane USP).

