
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
South Africa

DRUG PRODUCT COMPOSITIONS
Regulatory FDF Prices
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
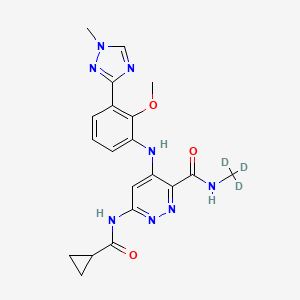

1. Bms-986165
1. Bms-986165
2. 1609392-27-9
3. Tyk2-in-4
4. Bms986165
5. Deucravacitinib [usan]
6. N0a21n6rau
7. 6-(cyclopropanecarbonylamino)-4-[2-methoxy-3-(1-methyl-1,2,4-triazol-3-yl)anilino]-n-(trideuteriomethyl)pyridazine-3-carboxamide
8. Deucravacitinib (usan)
9. Sotyktu
10. Unii-n0a21n6rau
11. Deucravacitinib [inn]
12. Deucravacitinib [jan]
13. Chembl4435170
14. Deucravacitinib [who-dd]
15. Schembl20520348
16. Gtpl10432
17. Ex-a3154
18. Bdbm50507816
19. Mfcd31715455
20. Nsc825520
21. S8879
22. Tyk2-in-4(bms986165)
23. Who 11342
24. At18623
25. Nsc-825520
26. Compound 11 [pmid: 31318208}
27. Ncgc00687789-01
28. Ac-31543
29. Hy-117287
30. Cs-0065044
31. D11817
32. 3-pyridazinecarboxamide, 6-((cyclopropylcarbonyl)amino)-4-((2-methoxy-3-(1-methyl-1h-1,2,4-triazol-3-yl)phenyl)amino)-n-(methyl-d3)-
33. 3-pyridazinecarboxamide, 6-((cyclopropylcarbonyl)amino]-4-((2-methoxy-3-(1-methyl-1h-1,2,4-triazol-3-yl)phenyl)amino)n-(methyl-d3)-
34. 6-((cyclopropylcarbonyl)amino)-4-((2-methoxy-3-(1-methyl-1h-1,2,4-triazol-3-yl)phenyl)amino)-n-(2h3)methyl-3-pyridazine-carboxamide
35. 6-((cyclopropylcarbonyl)amino]-4-((2-methoxy-3-(1-methyl-1h-1,2,4-triazol-3-yl)phenyl)amino)-n-((2h3)methyl)pyridazine-3-carboxamide
36. 6-[(cyclopropylcarbonyl)amino]-4-[[2-methoxy-3-(1-methyl-1h-1,2,4-triazol-3-yl)phenyl]amino]-n-(methyl-d3)-3-pyridazinecarboxamide
| Molecular Weight | 425.5 g/mol |
|---|---|
| Molecular Formula | C20H22N8O3 |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 7 |
| Exact Mass | 425.20031683 g/mol |
| Monoisotopic Mass | 425.20031683 g/mol |
| Topological Polar Surface Area | 136 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 648 |
| Isotope Atom Count | 3 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of psoriasis
Treatment of Systemic Lupus Erythematosus (SLE)
Dermatologic Agents
Drugs used to treat or prevent skin disorders or for the routine care of skin. (See all compounds classified as Dermatologic Agents.)
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
L - Antineoplastic and immunomodulating agents
L04 - Immunosuppressants
L04A - Immunosuppressants
L04AA - Selective immunosuppressants
L04AA56 - Deucravacitinib

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Sotyktu (deucravacitinib) is an oral, selective, allosteric TYK2 inhibitor. It inhibits the signaling of IL-23/12 and Type 1 IFN, cytokines involved in the pathogenesis of immune-mediated diseases.
Lead Product(s): Deucravacitinib
Therapeutic Area: Immunology Brand Name: Sotyktu
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable March 08, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Deucravacitinib
Therapeutic Area : Immunology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Bristol Myers Squibb Presents Late-Breaking Data from Phase 3 POETYK PsA-2 Trial
Details : Sotyktu (deucravacitinib) is an oral, selective, allosteric TYK2 inhibitor. It inhibits the signaling of IL-23/12 and Type 1 IFN, cytokines involved in the pathogenesis of immune-mediated diseases.
Product Name : Sotyktu
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
March 08, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Sotyktu (deucravacitinib) is an oral, selective, allosteric TYK2 inhibitor. It inhibits the signaling of IL-23/12 and Type 1 IFN, cytokines involved in the pathogenesis of immune-mediated diseases.
Lead Product(s): Deucravacitinib
Therapeutic Area: Immunology Brand Name: Sotyktu
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 23, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Deucravacitinib
Therapeutic Area : Immunology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Bristol Myers Squibb Announces Positive Results for Sotyktu in Psoriatic Arthritis
Details : Sotyktu (deucravacitinib) is an oral, selective, allosteric TYK2 inhibitor. It inhibits the signaling of IL-23/12 and Type 1 IFN, cytokines involved in the pathogenesis of immune-mediated diseases.
Product Name : Sotyktu
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
December 23, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Sotyktu (deucravacitinib) is an oral, selective, allosteric TYK2 inhibitor. It inhibits the signaling of IL-23/12 and Type 1 IFN, cytokines involved in the pathogenesis of immune-mediated diseases.
Lead Product(s): Deucravacitinib
Therapeutic Area: Dermatology Brand Name: BMS-986165
Study Phase: Phase IVProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 27, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Deucravacitinib
Therapeutic Area : Dermatology
Highest Development Status : Phase IV
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Bristol Myers Shows Sotyktu Efficacy in Scalp Psoriasis And Real-World Data
Details : Sotyktu (deucravacitinib) is an oral, selective, allosteric TYK2 inhibitor. It inhibits the signaling of IL-23/12 and Type 1 IFN, cytokines involved in the pathogenesis of immune-mediated diseases.
Product Name : BMS-986165
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
September 27, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Sotyktu (deucravacitinib) is an oral, selective, allosteric TYK2 inhibitor with a unique mechanism of action. It inhibits the signaling of IL-23, IL-12 and Type 1 IFN, key cytokines involved in the pathogenesis of moderate-to-severe plaque psoriasis.
Lead Product(s): Deucravacitinib
Therapeutic Area: Dermatology Brand Name: BMS-986165
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable November 10, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Deucravacitinib
Therapeutic Area : Dermatology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Sotyktu (deucravacitinib) is an oral, selective, allosteric TYK2 inhibitor with a unique mechanism of action. It inhibits the signaling of IL-23, IL-12 and Type 1 IFN, key cytokines involved in the pathogenesis of moderate-to-severe plaque psoriasis.
Product Name : BMS-986165
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
November 10, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Sotyktu (deucravacitinib) is an oral, selective, allosteric TYK2 inhibitor with a unique mechanism of action. It inhibits the signaling of IL-23, IL-12 and Type 1 IFN, key cytokines involved in the pathogenesis of multiple immune-mediated diseases.
Lead Product(s): Deucravacitinib
Therapeutic Area: Dermatology Brand Name: BMS-986165
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable March 28, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Deucravacitinib
Therapeutic Area : Dermatology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Sotyktu (deucravacitinib) is an oral, selective, allosteric TYK2 inhibitor with a unique mechanism of action. It inhibits the signaling of IL-23, IL-12 and Type 1 IFN, key cytokines involved in the pathogenesis of multiple immune-mediated diseases.
Product Name : BMS-986165
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
March 28, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Sotyktu (deucravacitinib) is an oral, selective, allosteric TYK2 inhibitor with a unique mechanism of action. It inhibits the signaling of IL-23, IL-12 and Type 1 IFN, key cytokines involved in the pathogenesis of multiple immune-mediated diseases.
Lead Product(s): Deucravacitinib
Therapeutic Area: Dermatology Brand Name: BMS-986165
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable January 27, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Deucravacitinib
Therapeutic Area : Dermatology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Sotyktu (deucravacitinib) is an oral, selective, allosteric TYK2 inhibitor with a unique mechanism of action. It inhibits the signaling of IL-23, IL-12 and Type 1 IFN, key cytokines involved in the pathogenesis of multiple immune-mediated diseases.
Product Name : BMS-986165
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
January 27, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Results from the POETYK PSO long-term extension (LTE) trial demonstrating durable efficacy and a consistent safety profile with BMS-986165 (deucravacitinib) treatment in adult patients with moderate to severe plaque psoriasis.
Lead Product(s): Deucravacitinib
Therapeutic Area: Dermatology Brand Name: BMS-986165
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 05, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Deucravacitinib
Therapeutic Area : Dermatology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Results from the POETYK PSO long-term extension (LTE) trial demonstrating durable efficacy and a consistent safety profile with BMS-986165 (deucravacitinib) treatment in adult patients with moderate to severe plaque psoriasis.
Product Name : BMS-986165
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
December 05, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Results add to the growing body of evidence and reinforce the efficacy profile of Sotyktu (deucravacitinib), a once-daily, oral, selective, allosteric tyrosine kinase 2 (TYK2) inhibitor for the treatment of moderate-to-severe plaque psoriasis.
Lead Product(s): Deucravacitinib
Therapeutic Area: Dermatology Brand Name: BMS-986165
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable October 09, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Deucravacitinib
Therapeutic Area : Dermatology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Bristol Myers Squibb Announces New Sotyktu™ (deucravacitinib) Long-Term Data Showing Clinical Ef...
Details : Results add to the growing body of evidence and reinforce the efficacy profile of Sotyktu (deucravacitinib), a once-daily, oral, selective, allosteric tyrosine kinase 2 (TYK2) inhibitor for the treatment of moderate-to-severe plaque psoriasis.
Product Name : BMS-986165
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
October 09, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Approval is based on results from pivotal Phase 3 trials, which demonstrated superior efficacy of once-daily Sotyktu (deucravacitinib) compared to placebo and twice-daily Otezla® (apremilast) in 1,684 patients aged 18 years and older with moderate-to-severe plaque psoriasis.
Lead Product(s): Deucravacitinib
Therapeutic Area: Dermatology Brand Name: BMS-986165
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 09, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Deucravacitinib
Therapeutic Area : Dermatology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
U.S. Food and Drug Administration Approves Sotyktu™ (deucravacitinib), Oral Treatment for Adults...
Details : Approval is based on results from pivotal Phase 3 trials, which demonstrated superior efficacy of once-daily Sotyktu (deucravacitinib) compared to placebo and twice-daily Otezla® (apremilast) in 1,684 patients aged 18 years and older with moderate-to-se...
Product Name : BMS-986165
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
September 09, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
BMS-986165 (Deucravacitinib), showed statistically significant efficacy at primary endpoint of Systemic Lupus Erythematosus (SLE) Responder Index-4 (SRI(4)) responses versus placebo at Week 32.
Lead Product(s): Deucravacitinib
Therapeutic Area: Immunology Brand Name: BMS-986165
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable January 06, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Deucravacitinib
Therapeutic Area : Immunology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : BMS-986165 (Deucravacitinib), showed statistically significant efficacy at primary endpoint of Systemic Lupus Erythematosus (SLE) Responder Index-4 (SRI(4)) responses versus placebo at Week 32.
Product Name : BMS-986165
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
January 06, 2022

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]CAS Number : 6228-73-5
End Use API : Deucravacitinib
About The Company : Founded with a mission to transform strategic capital into specialty chemicals, Ami Group focuses on Agrochemicals, Cosmetics, and Polymers. Ami Organics Ltd. i...

2-METHOXY-3-(1-METHYL-1H-1,2,4-TRIAZOL-3-YL)ANILIN...
CAS Number : 1609394-10-6
End Use API : Deucravacitinib
About The Company : Founded with a mission to transform strategic capital into specialty chemicals, Ami Group focuses on Agrochemicals, Cosmetics, and Polymers. Ami Organics Ltd. i...

2-Methoxy-3-(1-methyl-1H-1,2,4-triazol-3-yl)anilin...
CAS Number : 1609394-10-6
End Use API : Deucravacitinib
About The Company : Aventus Labs is a forward-thinking pharmaceutical company committed to developing and delivering high-quality, effective healthcare solutions. With a focus on r...

4,6-dihydroxypyridazine-3-carboxylic acid
CAS Number : 1442437-21-9
End Use API : Deucravacitinib
About The Company : LinkChem is a leading China headquartered CMO | CRO provider within the pharmaceutical industry. Our core focus includes: custom synthesis, process development,...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

Patents & EXCLUSIVITIES
Patent Expiration Date : 2033-11-07
US Patent Number : RE47929
Drug Substance Claim : Y
Drug Product Claim : Y
Application Number : 214958
Patent Use Code : U-3434
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2033-11-07

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE
93
PharmaCompass offers a list of Deucravacitinib API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Deucravacitinib manufacturer or Deucravacitinib supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Deucravacitinib manufacturer or Deucravacitinib supplier.
PharmaCompass also assists you with knowing the Deucravacitinib API Price utilized in the formulation of products. Deucravacitinib API Price is not always fixed or binding as the Deucravacitinib Price is obtained through a variety of data sources. The Deucravacitinib Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Deucravacitinib manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Deucravacitinib, including repackagers and relabelers. The FDA regulates Deucravacitinib manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Deucravacitinib API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Deucravacitinib manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Deucravacitinib supplier is an individual or a company that provides Deucravacitinib active pharmaceutical ingredient (API) or Deucravacitinib finished formulations upon request. The Deucravacitinib suppliers may include Deucravacitinib API manufacturers, exporters, distributors and traders.
click here to find a list of Deucravacitinib suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Deucravacitinib as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Deucravacitinib API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Deucravacitinib as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Deucravacitinib and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Deucravacitinib NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Deucravacitinib suppliers with NDC on PharmaCompass.
Deucravacitinib Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Deucravacitinib GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Deucravacitinib GMP manufacturer or Deucravacitinib GMP API supplier for your needs.
A Deucravacitinib CoA (Certificate of Analysis) is a formal document that attests to Deucravacitinib's compliance with Deucravacitinib specifications and serves as a tool for batch-level quality control.
Deucravacitinib CoA mostly includes findings from lab analyses of a specific batch. For each Deucravacitinib CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Deucravacitinib may be tested according to a variety of international standards, such as European Pharmacopoeia (Deucravacitinib EP), Deucravacitinib JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Deucravacitinib USP).
