
Synopsis
Synopsis
0
VMF
0
Australia

0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
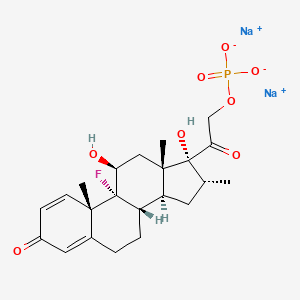

1. Decadron Phosphate
2. Dexamethasone 21-phosphate
3. Dexamethasone 21-phosphate, (6alpha,11beta,16alpha)-isomer
4. Dexamethasone 21-phosphate, Copper (+2) Salt (2:3), (11beta,16alpha)-isomer
5. Dexamethasone 21-phosphate, Disodium Salt, (11beta,16alpha)-isomer
6. Dexamethasone 21-phosphate, Disodium Salt, (6alpha,11beta,16alpha)-isomer
7. Dexamethasone 21-phosphate, Monosodium Salt, (11beta,16alpha)-isomer
8. Dexamethasone 21-phosphate, Sodium Salt, (11beta,16alpha)-isomer
9. Dexamethasone Phosphate
10. Dexamethasone Phosphate Disodium Salt
11. Dexamethasonedisodium Phosphate
12. Solu- Decadron
13. Spersadex
14. Spersadox
1. 2392-39-4
2. Dexamethasone 21-phosphate Disodium Salt
3. Dalalone
4. Dexadreson
5. Dexamethasone Disodium Phosphate
6. Megacort
7. Soldesam
8. Dexagro
9. 55203-24-2
10. Decadron Phosphate
11. Dexabene
12. Orgadrone
13. Ak-dex
14. Sodium Dexamethasone Phosphate
15. Dexamethasone 21-(disodium Phosphate)
16. Dexamethasone Phosphate Disodium
17. 2392-39-4 (disodium)
18. Chebi:4462
19. Tlc399
20. Tlc-399
21. Ai9376y64p
22. Nsc-756722
23. Disodium;[2-[(8s,9r,10s,11s,13s,14s,16r,17r)-9-fluoro-11,17-dihydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl]-2-oxoethyl] Phosphate
24. Spersadox
25. Decdan
26. Solu-decadron
27. Maxidex Ointment
28. Dsstox_cid_27429
29. Dsstox_rid_82342
30. Dsstox_gsid_47429
31. Hexadrol Injectable
32. Disodium [2-[(8s,9r,10s,11s,13s,14s,16r,17r)-9-fluoro-11,17-dihydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl]-2-oxoethyl] Phosphate
33. Colvasone
34. Dexagel
35. Onadron
36. Baldex
37. Mfcd00079105
38. Dexaject Sp
39. Egp 437
40. Sodium 2-((8s,9r,10s,11s,13s,14s,16r,17r)-9-fluoro-11,17-dihydroxy-10,13,16-trimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3h-cyclopenta[a]phenanthren-17-yl)-2-oxoethyl Phosphate
41. Cas-2392-39-4
42. Disodium Dexamethasone Phosphate
43. 21-disodium Phosphate Dexamethasone
44. Einecs 219-243-0
45. Dexamethasone-21-phosphate Disodium Salt
46. Dexamethazone Sodium Phosphate
47. Unii-ai9376y64p
48. Ncgc00094644-01
49. Dalalone (tn)
50. Mephamesone
51. Soludecadron
52. Totocortin
53. Solupen N
54. Egp437. Dex-phos
55. Dexamethasone Sodium Phosphate [usp:ban:jan]
56. Schembl7778
57. Decadron Inhalation, Injection, Ophthalmic Solution And Ointment, And Topical Cream
58. 9-fluoro-11beta,17,21-trihydroxy-16alpha-methylpregna-1,4-diene-3,20-dione 21-(dihydrogen Phosphate) Disodium Salt
59. Chembl2021430
60. Dtxsid3047429
61. Dexamethasone Phosphate Sodium Salt
62. Bcp16805
63. Tox21_113181
64. Tox21_302586
65. S4028
66. Akos015896357
67. Akos015951212
68. Am84812
69. Ccg-269820
70. Ks-1150
71. Nsc 756722
72. Ncgc00256783-01
73. Ac-17992
74. Dexamethasone Sodium Phosphate (jan/usp)
75. Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17-dihydroxy-16-methyl-21-(phosphonooxy)-, Disodium Salt, (11.beta.,16.alpha.)-
76. Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17-dihydroxy-16-methyl-21-(phosphonooxy)-, Disodium Salt, (11beta,16alpha)-
77. Pregna-1,4-diene-3,20-dione, 9-fluoro-11-beta,17,21-trihydroxy-16-alpha-methyl-, 21-(dihydrogen Phosphate) Disodium Salt
78. Dexamethasone Phosphate (as Sodium)
79. Dexamethasone Sodium Phosphate [jan]
80. B1588
81. Dexamethasone Sodium Phosphate [mart.]
82. Dexamethasone Sodium Phosphate [vandf]
83. En300-52795
84. C08175
85. D00975
86. Dexamethasone Sodium Phosphate [usp-rs]
87. Dexamethasone Sodium Phosphate [who-dd]
88. Dexamethasone Sodium Phosphate [who-ip]
89. A816989
90. A830522
91. Dexamethasone 21-phosphate Disodium Salt, >=98%
92. Dexamethasone Sodium Phosphate [green Book]
93. Dexamethasone Sodium Phosphate [orange Book]
94. Dexamethasone Sodium Phosphate [usp Impurity]
95. Dexamethasoni Natrii Phosphas [who-ip Latin]
96. Dexamethasone 21-phosphate Disodium Salt [mi]
97. Dexamethasone Sodium Phosphate [usp Monograph]
98. Q27106391
99. Neodecadron Component Dexamethasone Sodium Phosphate
100. Dexamethasone Sodium Phosphate Component Of Neodecadron
101. Dexamethasone Sodium Phosphate, British Pharmacopoeia (bp) Reference Standard
102. Dexamethasone Sodium Phosphate, European Pharmacopoeia (ep) Reference Standard
103. Dexamethasone Sodium Phosphate, United States Pharmacopeia (usp) Reference Standard
104. 9-fluoro-11.beta.,17,21-trihydroxy-16.alpha.-methylpregna-1,4-diene-3,20-dione 21-(dihydrogen Phosphate) Disodium Salt
105. Dexamethasone Sodium Phosphate For Peak Identification, European Pharmacopoeia (ep) Reference Standard
106. Dexamethasone Sodium Phosphate, Pharmaceutical Secondary Standard; Certified Reference Material
107. Disodium [2-[(8s,9r,10s,11s,13s,14s,16r,17r)-9-fluoranyl-10,13,16-trimethyl-11,17-bis(oxidanyl)-3-oxidanylidene-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl]-2-oxidanylidene-ethyl] Phosphate
108. Disodium 9-fluoro-11beta,17-dihydroxy-16alpha-methyl-3,20-dioxopregna-1,4-dien-21-yl Phosphate
109. Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17-dihydroxy-16-methyl-21-(phosphonooxy)-, Sodium Salt (1:2), (11beta,16alpha)-
| Molecular Weight | 516.4 g/mol |
|---|---|
| Molecular Formula | C22H28FNa2O8P |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 3 |
| Exact Mass | 516.13012157 g/mol |
| Monoisotopic Mass | 516.13012157 g/mol |
| Topological Polar Surface Area | 147 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 962 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 2 | |
|---|---|
| Drug Name | Dexamethasone sodium phosphate |
| Drug Label | Dexamethasone sodium phosphate is a water-soluble inorganic ester of dexamethasone. It occurs as a white or slightly yellow crystalline powder, is odorless or has a slight odor of alcohol, is exceedingly hygroscopic and is freely soluble in water.... |
| Active Ingredient | Dexamethasone sodium phosphate |
| Dosage Form | Injectable; Solution/drops |
| Route | Ophthalmic, otic; Injection |
| Strength | eq 10mg phosphate/ml; eq 0.1% phosphate; eq 4mg phosphate/ml |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa; Hikma Maple; Bausch And Lomb; Alcon Pharms; Luitpold; Agila Speclts |
| 2 of 2 | |
|---|---|
| Drug Name | Dexamethasone sodium phosphate |
| Drug Label | Dexamethasone sodium phosphate is a water-soluble inorganic ester of dexamethasone. It occurs as a white or slightly yellow crystalline powder, is odorless or has a slight odor of alcohol, is exceedingly hygroscopic and is freely soluble in water.... |
| Active Ingredient | Dexamethasone sodium phosphate |
| Dosage Form | Injectable; Solution/drops |
| Route | Ophthalmic, otic; Injection |
| Strength | eq 10mg phosphate/ml; eq 0.1% phosphate; eq 4mg phosphate/ml |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa; Hikma Maple; Bausch And Lomb; Alcon Pharms; Luitpold; Agila Speclts |
Glucocorticoids
A group of CORTICOSTEROIDS that affect carbohydrate metabolism (GLUCONEOGENESIS, liver glycogen deposition, elevation of BLOOD SUGAR), inhibit ADRENOCORTICOTROPIC HORMONE secretion, and possess pronounced anti-inflammatory activity. They also play a role in fat and protein metabolism, maintenance of arterial blood pressure, alteration of the connective tissue response to injury, reduction in the number of circulating lymphocytes, and functioning of the central nervous system. (See all compounds classified as Glucocorticoids.)
 Click Us!
Click Us! 
GDUFA
DMF Review : Reviewed
Rev. Date : 2013-01-28
Pay. Date : 2013-01-16
DMF Number : 3963
Submission : 1980-10-10
Status : Active
Type : II

Certificate Number : R2-CEP 1992-014 - Rev 07
Issue Date : 2021-12-16
Type : Chemical
Substance Number : 549
Status : Valid

Registration Number : 222MF10092
Registrant's Address : 82 Avenue Raspail 94250 Gentilly France
Initial Date of Registration : 2010-03-17
Latest Date of Registration : --

NDC Package Code : 82298-113
Start Marketing Date : 2011-02-07
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : BASH HEALTH KOREA CORP.
Registration Date : 2023-01-06
Registration Number : 20230106-209-J-1432
Manufacturer Name : EUROAPI France
Manufacturer Address : 4 La Paterie, 63480 VERTOLAYE, France

| Available Reg Filing : ASMF, IN, RU, ROW |

GDUFA
DMF Review : Reviewed
Rev. Date : 2022-03-04
Pay. Date : 2022-02-28
DMF Number : 6525
Submission : 1986-08-11
Status : Active
Type : II

Certificate Number : R1-CEP 1998-154 - Rev 06
Issue Date : 2021-07-07
Type : Chemical
Substance Number : 549
Status : Valid

Date of Issue : 2022-08-31
Valid Till : 2025-07-02
Written Confirmation Number : WC-0162
Address of the Firm :
 Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
 Faran Shimi: Leading producer of high-quality APIs & alkaloid opiates, serving major pharmaceutical companies across the Middle East.
Faran Shimi: Leading producer of high-quality APIs & alkaloid opiates, serving major pharmaceutical companies across the Middle East.
 Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.
Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.

Registration Number : 222MF10093
Registrant's Address : 15 rue Traversie(\')re 75012 Paris France
Initial Date of Registration : 2010-03-17
Latest Date of Registration : --

NDC Package Code : 82298-113
Start Marketing Date : 2011-02-07
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : BASH HEALTH KOREA CORP.
Registration Date : 2023-01-06
Registration Number : 20230106-209-J-1432
Manufacturer Name : EUROAPI France
Manufacturer Address : 4 La Paterie, 63480 VERTOLAYE, France

| Available Reg Filing : ASMF, IN, RU, ROW |

Date of Issue : 2022-07-05
Valid Till : 2025-07-02
Written Confirmation Number : WC-0161A2
Address of the Firm :
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : Complete
Rev. Date : 2013-01-28
Pay. Date : 2013-01-16
DMF Number : 3963
Submission : 1980-10-10
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2022-03-04
Pay. Date : 2022-02-28
DMF Number : 6525
Submission : 1986-08-11
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 2378
Submission : 1974-12-18
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 3684
Submission : 1980-01-03
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 4497
Submission : 1982-03-15
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 3841
Submission : 1980-05-21
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 582
Submission : 1963-06-11
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 5971
Submission : 1985-08-07
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dexamethasone metasulfobenzoate sodium
Registration Number : 222MF10093
Registrant's Address : 15 rue Traversie(')re 75012 Paris France
Initial Date of Registration : 2010-03-17
Latest Date of Registration : 2016-03-07
Dexamethasone sodium phosphate
Registration Number : 222MF10092
Registrant's Address : 82 Avenue Raspail 94250 Gentilly France
Initial Date of Registration : 2010-03-17
Latest Date of Registration : 2020-09-14
Registration Number : 221MF10245
Registrant's Address : Viale Spagna, 156 Cologno Monzese (MI) - ITALY
Initial Date of Registration : 2009-11-16
Latest Date of Registration : 2009-11-16

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dexamethasone disodium phosphate
Registrant Name : BASH HEALTH KOREA CORP.
Registration Date : 2023-01-06
Registration Number : 20230106-209-J-1432
Manufacturer Name : EUROAPI France
Manufacturer Address : 4 La Paterie, 63480 VERTOLAYE, France
Dexamethasone sodium phosphate
Registrant Name : Kyungdong Pharmaceutical Co., Ltd.
Registration Date : 2023-03-23
Registration Number : 20210310-209-J-863(2)
Manufacturer Name : Symbiotica Specialty Ingredi...
Manufacturer Address : No. 518, Jalan Waja 4, Taman Industri Waja, 09000 Kulim, Kedah, Malaysia@No. 19, Xiny...

Dexamethasone sodium phosphate
Registrant Name : Wonpung Pharmaceutical Co., Ltd.
Registration Date : 2022-08-08
Registration Number : 20210310-209-J-863(1)
Manufacturer Name : Symbiotica Specialty Ingredi...
Manufacturer Address : No. 518, Jalan Waja 4, Taman Industri Waja, 09000 Kulim, Kedah, Malaysia@No. 19, Xiny...

Dexamethasone sodium phosphate
Registrant Name : Insung Trading Co., Ltd.
Registration Date : 2021-03-10
Registration Number : 20210310-209-J-863
Manufacturer Name : Symbiotica Specialty Ingredi...
Manufacturer Address : No. 518, Jalan Waja 4, Taman Industri Waja, 09000 Kulim, Kedah, Malaysia@No. 19, Xiny...

Dexamethasone sodium phosphate
Registrant Name : Ace Biopharm Co., Ltd.
Registration Date : 2021-03-16
Registration Number : 20210316-209-J-887
Manufacturer Name : Tianjin Tianyao Pharmaceutic...
Manufacturer Address : No.19 Xinye 9th Street, west Area of Tianjin Economic-Technological Development...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Semnur’s lead program, Semdexa (dexamethasone sodium phosphate), is the first non-opioid novel injectable corticosteroid gel formulation for moderate to severe chronic radicular sciatica.
Lead Product(s): Dexamethasone Sodium Phosphate
Therapeutic Area: Neurology Brand Name: Semdexa
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Denali Capital Acquisition Corp
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Merger September 03, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Dexamethasone Sodium Phosphate
Therapeutic Area : Neurology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Denali Capital Acquisition Corp
Deal Size : Undisclosed
Deal Type : Merger
Semnur to go Public via SPAC Merger with Denali Capital in $2.5 bln Deal
Details : Semnur’s lead program, Semdexa (dexamethasone sodium phosphate), is the first non-opioid novel injectable corticosteroid gel formulation for moderate to severe chronic radicular sciatica.
Brand Name : Semdexa
Molecule Type : Small molecule
Upfront Cash : Undisclosed
September 03, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
EryDex (dexamethasone sodium phosphate) is a glucocorticoid receptor agonist, which is being evaluated in the late-stage clinical trial studies for the treatment of Ataxia-Telangiectasia.
Lead Product(s): Dexamethasone Sodium Phosphate
Therapeutic Area: Genetic Disease Brand Name: EryDex
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 25, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Dexamethasone Sodium Phosphate
Therapeutic Area : Genetic Disease
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Quince Announces First Patient Dosed in Phase 3 Trial of EryDex for Ataxia-Telangiectasia
Details : EryDex (dexamethasone sodium phosphate) is a glucocorticoid receptor agonist, which is being evaluated in the late-stage clinical trial studies for the treatment of Ataxia-Telangiectasia.
Brand Name : EryDex
Molecule Type : Small molecule
Upfront Cash : Not Applicable
June 25, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
EryDex (dexamethasone sodium phosphate), utilizing company's AIDE technology, is in Phase 3 for treating Ataxia-Telangiectasia, a rare pediatric condition.
Lead Product(s): Dexamethasone Sodium Phosphate
Therapeutic Area: Rare Diseases and Disorders Brand Name: EryDex
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 03, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Dexamethasone Sodium Phosphate
Therapeutic Area : Rare Diseases and Disorders
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Quince Therapeutics Receives U.S. FDA Fast Track Designation for EryDex System
Details : EryDex (dexamethasone sodium phosphate), utilizing company's AIDE technology, is in Phase 3 for treating Ataxia-Telangiectasia, a rare pediatric condition.
Brand Name : EryDex
Molecule Type : Small molecule
Upfront Cash : Not Applicable
June 03, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
SP-102 (semdexatm) is expected to be administered in up to 3 injections during a 6-month observation period. Completion of enrollment for Lumbosacral Radicular Pain (Sciatica) in the trial is projected to occur in 2025.
Lead Product(s): Dexamethasone Sodium Phosphate
Therapeutic Area: Musculoskeletal Brand Name: SP-102
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable November 02, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Dexamethasone Sodium Phosphate
Therapeutic Area : Musculoskeletal
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : SP-102 (semdexatm) is expected to be administered in up to 3 injections during a 6-month observation period. Completion of enrollment for Lumbosacral Radicular Pain (Sciatica) in the trial is projected to occur in 2025.
Brand Name : SP-102
Molecule Type : Small molecule
Upfront Cash : Not Applicable
November 02, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Through the acquisition, Quince gains right for EryDel's Phase 3 lead asset, EryDex (dexamethasone sodium phosphate), targeting a rare fatal pediatric neurological disease, Ataxia-Telangiectasia (A-T), which currently has no approved treatments.
Lead Product(s): Dexamethasone Sodium Phosphate
Therapeutic Area: Genetic Disease Brand Name: EryDex
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Quince Therapeutics
Deal Size: $485.0 million Upfront Cash: $485.0 million
Deal Type: Acquisition October 23, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Dexamethasone Sodium Phosphate
Therapeutic Area : Genetic Disease
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Quince Therapeutics
Deal Size : $485.0 million
Deal Type : Acquisition
Quince Therapeutics Completes Acquisition of EryDel S.p.A.
Details : Through the acquisition, Quince gains right for EryDel's Phase 3 lead asset, EryDex (dexamethasone sodium phosphate), targeting a rare fatal pediatric neurological disease, Ataxia-Telangiectasia (A-T), which currently has no approved treatments.
Brand Name : EryDex
Molecule Type : Small molecule
Upfront Cash : $485.0 million
October 23, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
EryDex (dexamethasone sodium phosphate) utilizes a unique drug/device combination that enables a fully automated process at the point of patient care for the autologous intracellular drug encapsulation for the Treatment of Ataxia-Telangiectasia.
Lead Product(s): Dexamethasone Sodium Phosphate
Therapeutic Area: Genetic Disease Brand Name: EryDex
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable September 28, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Dexamethasone Sodium Phosphate
Therapeutic Area : Genetic Disease
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
U.S. FDA Partial Clinical Hold Lifted on IND for EryDel’s Lead Phase 3 Asset EryDex for the Trea...
Details : EryDex (dexamethasone sodium phosphate) utilizes a unique drug/device combination that enables a fully automated process at the point of patient care for the autologous intracellular drug encapsulation for the Treatment of Ataxia-Telangiectasia.
Brand Name : EryDex
Molecule Type : Small molecule
Upfront Cash : Not Applicable
September 28, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
AVM0703 is small molecule immunomodulatory drug enrolling Phase 2 trials in US in relapsed/refractory Non-Hodgkin's Lymphoma (NHL) which began enrollment Q3 2023 (partially funded by NCI Ph II FastTrak grant 1R44CA272096).
Lead Product(s): Dexamethasone Sodium Phosphate
Therapeutic Area: Oncology Brand Name: AVM0703
Study Phase: Phase IProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 01, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Dexamethasone Sodium Phosphate
Therapeutic Area : Oncology
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : AVM0703 is small molecule immunomodulatory drug enrolling Phase 2 trials in US in relapsed/refractory Non-Hodgkin's Lymphoma (NHL) which began enrollment Q3 2023 (partially funded by NCI Ph II FastTrak grant 1R44CA272096).
Brand Name : AVM0703
Molecule Type : Small molecule
Upfront Cash : Not Applicable
August 01, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Through the acquisition, Quince gains right for EryDel's Phase 3 lead asset, EryDex (dexamethasone sodium phosphate), targeting a rare fatal pediatric neurological disease, Ataxia-Telangiectasia (A-T), which currently has no approved treatments.
Lead Product(s): Dexamethasone Sodium Phosphate
Therapeutic Area: Genetic Disease Brand Name: EryDex
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Quince Therapeutics
Deal Size: $485.0 million Upfront Cash: $485.0 million
Deal Type: Acquisition July 24, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Dexamethasone Sodium Phosphate
Therapeutic Area : Genetic Disease
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Quince Therapeutics
Deal Size : $485.0 million
Deal Type : Acquisition
Details : Through the acquisition, Quince gains right for EryDel's Phase 3 lead asset, EryDex (dexamethasone sodium phosphate), targeting a rare fatal pediatric neurological disease, Ataxia-Telangiectasia (A-T), which currently has no approved treatments.
Brand Name : EryDex
Molecule Type : Small molecule
Upfront Cash : $485.0 million
July 24, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
AVM0703 (Dexamethasone sodium phosphate) is a small molecule which triggers the production and release of endogenous bispecific gamma delta TCR+ invariant TCR+ Natural Killer T-like cells (AVM-NKT).
Lead Product(s): Dexamethasone Sodium Phosphate
Therapeutic Area: Oncology Brand Name: AVM0703
Study Phase: Phase I/ Phase IIProduct Type: Small molecule
Sponsor: National Cancer Institute
Deal Size: $2.0 million Upfront Cash: Undisclosed
Deal Type: Funding June 14, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Dexamethasone Sodium Phosphate
Therapeutic Area : Oncology
Highest Development Status : Phase I/ Phase II
Partner/Sponsor/Collaborator : National Cancer Institute
Deal Size : $2.0 million
Deal Type : Funding
Details : AVM0703 (Dexamethasone sodium phosphate) is a small molecule which triggers the production and release of endogenous bispecific gamma delta TCR+ invariant TCR+ Natural Killer T-like cells (AVM-NKT).
Brand Name : AVM0703
Molecule Type : Small molecule
Upfront Cash : Undisclosed
June 14, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Under the terms of the agreement, TLC will primarily be responsible for the development of the product TLC599 (Dexamethasone Sodium Phosphate) and EVL will primarily be responsible for obtaining regulatory approval and commercialization of the product in the United States.
Lead Product(s): Dexamethasone Sodium Phosphate
Therapeutic Area: Rheumatology Brand Name: TLC599
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Endo International
Deal Size: $140.0 million Upfront Cash: $30.0 million
Deal Type: Agreement June 13, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Dexamethasone Sodium Phosphate
Therapeutic Area : Rheumatology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Endo International
Deal Size : $140.0 million
Deal Type : Agreement
Details : Under the terms of the agreement, TLC will primarily be responsible for the development of the product TLC599 (Dexamethasone Sodium Phosphate) and EVL will primarily be responsible for obtaining regulatory approval and commercialization of the product in...
Brand Name : TLC599
Molecule Type : Small molecule
Upfront Cash : $30.0 million
June 13, 2022

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
DEXAMETHASONE SODIUM PHOSPHATE
Brand Name : HEXADROL
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 4MG PHOSPHATE/ML **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Packaging :
Approval Date : 1982-01-01
Application Number : 14694
Regulatory Info : DISCN
Registration Country : USA
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
DEXAMETHASONE SODIUM PHOSPHATE
Brand Name : HEXADROL
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 10MG PHOSPHATE/ML **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Packaging :
Approval Date : 1982-01-01
Application Number : 14694
Regulatory Info : DISCN
Registration Country : USA
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
DEXAMETHASONE SODIUM PHOSPHATE
Brand Name : HEXADROL
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 20MG PHOSPHATE/ML
Packaging :
Approval Date : 1982-01-01
Application Number : 14694
Regulatory Info : DISCN
Registration Country : USA
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Dexamethasone sodium phosphate
Brand Name : Voxidex
Dosage Form : EED
Dosage Strength : 1mg/ml
Packaging : 1X1mg/ml
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
Regulatory Info : DISCN
Registration Country : USA
DEXAMETHASONE SODIUM PHOSPHATE
Brand Name : DEXAMETHASONE SODIUM PHOSPHATE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 4MG PHOSPHATE/ML
Packaging :
Approval Date : 1986-04-09
Application Number : 89169
Regulatory Info : DISCN
Registration Country : USA
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
DEXAMETHASONE SODIUM PHOSPHATE
Brand Name : DEXAMETHASONE SODIUM PHOSPHATE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 4MG PHOSPHATE/ML
Packaging :
Approval Date : 1982-01-01
Application Number : 84355
Regulatory Info : DISCN
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
DEXAMETHASONE SODIUM PHOSPHATE
Brand Name : DEXAMETHASONE SODIUM PHOSPHATE PRESERVATIVE FREE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 10MG PHOSPHATE/ML
Packaging :
Approval Date : 2003-04-11
Application Number : 40491
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
DEXAMETHASONE SODIUM PHOSPHATE
Brand Name : DEXAMETHASONE SODIUM PHOSPHATE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 4MG PHOSPHATE/ML
Packaging :
Approval Date : 1982-07-21
Application Number : 87440
Regulatory Info : DISCN
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
DEXAMETHASONE SODIUM PHOSPHATE
Brand Name : DECADRON
Dosage Form : CREAM;TOPICAL
Dosage Strength : EQ 0.1% PHOSPHATE
Packaging :
Approval Date : 1982-01-01
Application Number : 11983
Regulatory Info : DISCN
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : OTC
Registration Country : Canada
DEXAMETHASONE SODIUM PHOSPHATE
Brand Name : DEXACORT 5
Dosage Form : SOLUTION
Dosage Strength : 5MG/ML
Packaging : 50ML/100ML
Approval Date :
Application Number : 2314118
Regulatory Info : OTC
Registration Country : Canada

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Dexamethasone Sodium Phosphate
Brand Name :
Dosage Form : Injection
Dosage Strength : 2ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dexamethasone Sodium Phosphate
Dosage : Injection
Dosage Strength : 2ML
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Dexamethasone Sodium Phosphate
Brand Name :
Dosage Form : Injection
Dosage Strength : 4MG/ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dexamethasone Sodium Phosphate
Dosage : Injection
Dosage Strength : 4MG/ML
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name :
Dosage Form : Injection
Dosage Strength :
Packaging : 5ml & 10ml Multi dose vial
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : 5ml & 10ml Multi dose vial
Regulatory Info : Generic
Dosage : Injection
Dosage Strength :
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Dexamethasone Sodium Phosphate
Brand Name :
Dosage Form : Injection
Dosage Strength : 4MG/ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dexamethasone Sodium Phosphate
Dosage : Injection
Dosage Strength : 4MG/ML
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Dexamethasone Sodium Phosphate
Brand Name : DECADRON
Dosage Form : Injection
Dosage Strength : 8MG/2ML
Packaging : 3 ampoules, 6 ampoules
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : 3 ampoules, 6 ampoules
Regulatory Info :
Dexamethasone Sodium Phosphate
Dosage : Injection
Dosage Strength : 8MG/2ML
Brand Name : DECADRON
Approval Date :
Application Number :
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Dexamethasone Sodium Phosphate
Brand Name : DECADRON
Dosage Form : Injection
Dosage Strength : 2MG/ML
Packaging : 10 ml bottle, 30 ml bottle
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : 10 ml bottle, 30 ml bottle
Regulatory Info :
Dexamethasone Sodium Phosphate
Dosage : Injection
Dosage Strength : 2MG/ML
Brand Name : DECADRON
Approval Date :
Application Number :
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Dexamethasone Sodium Phosphate
Brand Name :
Dosage Form : Injection
Dosage Strength : 150mg/4ml
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dexamethasone Sodium Phosphate
Dosage : Injection
Dosage Strength : 150mg/4ml
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Dexamethasone Sodium Phosphate
Brand Name :
Dosage Form : Injection
Dosage Strength : 4MG/ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dexamethasone Sodium Phosphate
Dosage : Injection
Dosage Strength : 4MG/ML
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Dexamethasone Sodium Phosphate
Brand Name :
Dosage Form : Injection
Dosage Strength : 4MG/ML
Packaging : 2ml Ampoule
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : 2ml Ampoule
Regulatory Info :
Dexamethasone Sodium Phosphate
Dosage : Injection
Dosage Strength : 4MG/ML
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name : EUDEX
Dosage Form : INJECTION
Dosage Strength : 4MG/ML
Packaging : 2ml amp ; vial
Approval Date :
Application Number : 89280
Regulatory Info : Generic
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : 2ml amp ; vial
Regulatory Info : Generic
Dosage : INJECTION
Dosage Strength : 4MG/ML
Brand Name : EUDEX
Approval Date :
Application Number : 89280
Registration Country : India

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Dosage Form : Cream / Lotion / Ointment
Grade : Topical
Category : Emulsifying Agents, Solubilizers, Surfactant & Foaming Agents, Topical
Dosage Form : Capsule, Cream / Lotion / Ointment, Emulsion, Gel, Injectable / Parenteral, Suspension, Tablet
Grade : Parenteral, Oral, Topical
Category : Emulsifying Agents, Film Formers & Plasticizers, Parenteral, Solubilizers, Surfactant & Foaming Agents, Thickeners and Stabilizers, Topical
Application : Emulsifying Agents, Film Formers & Plasticizers, Parenteral, Solubilizers, Surfactant & Foaming Agents, Thickeners and Stabilizers, Topical
Excipient Details : Polysorbate 80 is used as a plasticizer, solubilizer, emulsifier, surfactant, and suspension stabilizer. It is also used in parenteral products.
Dosage Form : Cream / Lotion / Ointment, Gel, Injectable / Parenteral, Tablet
Grade : Parenteral, Oral, Topical
Category : Parenteral, Solubilizers, Topical
Brand Name : Benzalkonium Chloride 17% USP NF
Application : Solubilizers
Excipient Details : A&C’s Benzalkonium Chloride 17% is a preservative which meets the NF monograph.
Pharmacopoeia Ref : USP NF
Technical Specs : 17% USP NF
Ingredient(s) : Benzalkonium chloride excipient
Brand Name : Benzalkonium Chloride 50% NF
Application : Solubilizers
Excipient Details : A&C’s Benzalkonium Chloride 50% is a preservative which meets the NF monograph. It acts as a quarternary ammonium salt.
Dosage Form : Capsule, Cream / Lotion / Ointment, Suspension, Tablet
Grade : Oral, Topical & Parenteral
Category : Solubilizers, Surfactant & Foaming Agents
Application : Solubilizers, Surfactant & Foaming Agents
Excipient Details : Polysorbate 80 acts as solubilizer, emulsifier and wetting agent.
Dosage Form : Gel, Softgel Capsule, Solution, Suppository
Grade : Not Available
Category : Solubilizers
Application : Solubilizers
Excipient Details : Nonionic solubilizer, emulsifier and co-emulsifier
Dosage Form : Cream / Lotion / Ointment, Injectable / Parenteral
Grade : Parenteral and Topical
Category : Parenteral, Topical
Application : Parenteral, Topical
Dosage Form : Capsule, Cream / Lotion / Ointment, Emulsion, Gel, Paste, Shampoo, Solution, Syrup, Tablet
Grade : Topical, Oral
Category : Film Formers & Plasticizers, Solubilizers, Surfactant & Foaming Agents, Topical
Brand Name : MONTANOX 80 PHA PREMIUM
Application : Film Formers & Plasticizers, Solubilizers, Surfactant & Foaming Agents, Topical
Excipient Details : Non-Ionic Hydrophilic Surfactant, Emulsifier, Solubilizer
Pharmacopoeia Ref : Ph.Eur, USP-NF
Technical Specs : HLB: 15, EO: 20; EXCiPACT
Ingredient(s) : Polysorbate 80
Brand Name : Polysorbate 80 Multi-Compendial
Application : Solubilizers
Excipient Details : A & C's Polysorbate 80 multi-compendial is an excipient which meets USP-NF, EP, BP and JP monographs.
Global Sales Information

Market Place

Reply
21 Aug 2024
Reply
26 Oct 2023
Reply
26 Jul 2023
Reply
24 Jul 2023
Reply
29 Mar 2023
Reply
11 Feb 2023
Reply
02 Sep 2022
Reply
29 Jul 2022
Reply
20 Nov 2021
Reply
25 Sep 2021
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Reply
07 Jul 2023
Reply
09 Mar 2023
Reply
23 Apr 2022
Reply
29 Nov 2021
Reply
24 Aug 2021
Reply
12 Jul 2021
Reply
14 Jun 2021
Reply
22 Mar 2021
Reply
13 Sep 2018
Reply
12 Oct 2017
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
REF. STANDARDS & IMPURITIES

CAS Number : 125-02-0
Quantity Per Vial : 100 mg
Sale Unit : 1
Order Code : P2810000
Batch No : 4
Price (€) : 79
Storage : +5°C ± 3°C

Dexamethasone sodium phosphate for peak identification
CAS Number : 2392-39-4
Quantity Per Vial : 10 mg
Sale Unit : 1
Order Code : Y0001477
Batch No : 1
Price (€) : 79
Storage : +5°C ± 3°C

Dexamethasone sodium phosphate
CAS Number : 2392-39-4
Quantity Per Vial : 130 mg
Sale Unit : 1
Order Code : D0720000
Batch No : 7
Price (€) : 79
Storage : +5°C ± 3°C

Betamethasone sodium phosphate
CAS Number : 151-73-5
Quantity Per Vial : 100 mg
Sale Unit : 1
Order Code : B1045000
Batch No : 4
Price (€) : 79
Storage : +5°C ± 3°C

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dexamethasone Sodium Phosphate (500 mg)
CAS Number : 2392-39-4
Quantity Per Vial :
Price ($) : 230
Catalog Number : 1177032
Current Lot : R06110
Previous Lot : F0M447 (31-JAN-2018)
NDC Code :

Dexamethasone Phosphate (500 mg)
CAS Number : 312-93-6
Quantity Per Vial :
Price ($) : 230
Catalog Number : 1177000
Current Lot : L2M534
Previous Lot : L1M534 (31-AUG-2018)
NDC Code :

Dexamethasone Phosphate (500 mg)
CAS Number : 312-93-6
Quantity Per Vial : 500
Sale Unit : mg
Price : $245.00
Details : Material Origin- Chemical Synthesis; USMCA- N...
Monograph :
Storage :
Code/Batch No : Catalog #1177000 / R107K0

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?