
Synopsis
Synopsis
0
EU WC
0
KDMF
0
VMF
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
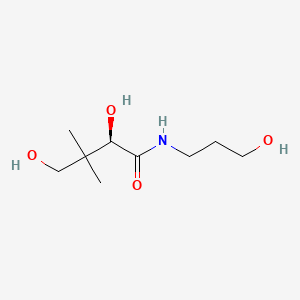

1. (+)-2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethylbutyramide
2. 2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethylbutanamide
3. Bepanthen
4. Butanamide, 2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethyl-, (+--)-
5. Corneregel
6. D-panthenol
7. Dexpanthenol Heumann
8. Dl-panthenol
9. Ilopan
10. Marolderm
11. Nasenspray Ratiopharm Panthenol
12. Nasicur
13. Otriven Dexpanthenol
14. Pan Rhinol
15. Pan-ophtal
16. Panthenol
17. Panthenol Braun
18. Panthenol Jenapharm
19. Panthenol Law
20. Panthenol Lichtenstein
21. Panthenol Von Ct
22. Panthenol-ratiopharm
23. Panthoderm
24. Panthogenat
25. Pantothenol
26. Repa-ophtal
27. Rhinoclir
28. Siozwo Sana
29. Ucee D
30. Urupan
31. Wund- Und Heilsalbe Law
1. D-panthenol
2. 81-13-0
3. Pantothenol
4. Ilopan
5. D-pantothenyl Alcohol
6. Bepanthen
7. Pantol
8. Bepanthene
9. Bepantol
10. (+)-panthenol
11. Provitamin B
12. Motilyn
13. Panadon
14. Panthoderm
15. Thenalton
16. Zentinic
17. D-pantothenol
18. Pantothenyl Alcohol
19. Cozyme
20. D-p-a Injection
21. D(+)-panthenol
22. Pantenyl
23. Synapan
24. Urupan
25. D(+)-pantothenyl Alcohol
26. Intrapan
27. D Panthenol
28. D-panthenol 50
29. Provitamin B5
30. Pantothenylol
31. (2r)-2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethylbutanamide
32. Dexpantenol
33. (r)-2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethylbutanamide
34. Dextro Pantothenyl Alcohol
35. Propanolamine, N-pantoyl-
36. Dexpanthenolum
37. Alcopan-250
38. Penthenol
39. Varitan
40. N-pantoyl-propanolamine
41. Panthenol (d)
42. Butanamide, 2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethyl-, (2r)-
43. Panthenol (jan)
44. Prestwick_529
45. Dexpanthenol (1.20 G/ml)
46. Ilopan (tn)
47. D-(+)-panthenol
48. Panthenol, (+)-
49. D-(+)-2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethylbutyramide
50. N-pantoyl-3-propanolamine
51. Nsc 302962
52. D-(+)-pantothenyl Alcohol
53. 1o6c93ri7z
54. Chebi:27373
55. Butanamide, 2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethyl-, (r)-
56. Pro-itamin B5
57. Butyramide, 2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethyl-, D-(+)-
58. Component Of Pantho-f
59. Ncgc00142622-03
60. Panthenol [jan]
61. 2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethylbutanamide, (r)-
62. 2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethylbutyramide, D-(+)-
63. Dsstox_cid_2906
64. Dsstox_rid_76783
65. Dsstox_gsid_22906
66. Panthenolum
67. Pantenol
68. Pantenolo
69. Sinecort
70. (r)-2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethylbutyramide
71. D(+)-2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethylbutyramide
72. Component Of Zentinic
73. Pantothenol, D-
74. Alcool Dl-pantotenilico
75. (+-)-pantothenyl Alcohol
76. Dexpanthenol [usan]
77. Dexpantenol [inn-spanish]
78. Dexpanthenolum [inn-latin]
79. Unii-1o6c93ri7z
80. (r)-2,4-dihydroxy-3,3-dimethylbutyric 3-hydroxypropylamide
81. Dexpanthenol;
82. Ccris 3947
83. Hsdb 296
84. Nsc302962
85. Fancol Dl
86. Nsc-302962
87. Cas-81-13-0
88. Ncgc00186658-01
89. Dexpanthenol [usan:usp:inn:ban]
90. Einecs 201-327-3
91. Dexpanthenol (usp)
92. Mfcd00065006
93. Cornergel
94. Dolobene
95. Bay 81-2996
96. Brn 1724947
97. Panthenol, (+ )-
98. Panthenol 50w
99. Prestwick0_000022
100. Prestwick1_000022
101. Prestwick2_000022
102. Prestwick3_000022
103. Dexpanthenol (usp/inn)
104. Dexpanthenol [mi]
105. D-panthenol Usp/bp/ip
106. Compnent Of Ilopan-choline
107. Dexpanthenol [fcc]
108. Dexpanthenol [inn]
109. Bmse000445
110. Panthenol, (r)-
111. Dexpanthenol [hsdb]
112. Ec 201-327-3
113. Alcopan 250
114. D-panthenol [vandf]
115. Dexpanthenol [vandf]
116. Schembl15861
117. Bspbio_000083
118. Dexpanthenol [mart.]
119. 4-04-00-01652 (beilstein Handbook Reference)
120. Dexpanthenol [usp-rs]
121. Dexpanthenol [who-dd]
122. Spbio_002004
123. Bpbio1_000093
124. Chembl1200979
125. Dexpanthenol - Usp/fcc Kosher
126. Dtxsid3022906
127. Schembl20553090
128. Dexpanthenol [orange Book]
129. Hms1568e05
130. Hms2094e09
131. Hms2095e05
132. Hms3712e05
133. Dexpanthenol [ep Monograph]
134. (r)-2,4-dihydroxy-n-(3-hydroxy-propyl)-3,3-dimethylbutanamide
135. Hy-b1391
136. Zinc1530303
137. D-panthenol, >=98.0% (nt)
138. Dexpanthenol [usp Monograph]
139. Tox21_111563
140. Lmfa08020198
141. S4695
142. Akos015841507
143. Akos015901947
144. Calcium D-pantothenate Usp/bp/ep/ip
145. D(+)-alpha,gamma-dihydroxy-n-(3-hydroxypropyl)-beta,beta-dimethylbutyramide
146. Tox21_111563_1
147. Ccg-213429
148. Cs-8175
149. Db09357
150. Alpha,gamma-dihydroxy-n-(3-hydroxypropyl)-beta,beta-dimethylbutyramide, D-(+)-
151. Ncgc00142622-01
152. Ncgc00142622-04
153. As-14732
154. D-panthenol 10 Microg/ml In Acetonitrile
155. Dexpanthenol, Tested According To Ph.eur.
156. Sbi-0206936.p001
157. D-panthenol, Vetec(tm) Reagent Grade, 98%
158. P0692
159. C05944
160. D00193
161. D70909
162. Q-201048
163. Q47495755
164. A6cf1a81-5b98-4c28-a379-ea28fa9dd210
165. (r)-3-(2,4-dihydroxy-3,3-dimethylbutyramido)-1-propanol
166. Dexpanthenol, European Pharmacopoeia (ep) Reference Standard
167. (2r)-2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethyl-butanamide
168. (r)-()-2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethylbutyramide
169. (r)-(+)-2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethylbutanamide
170. (r)-2,4-dihydroxy-3,3-dimethyl-n-(3-hydroxypropyl)-butanamide
171. (r)-2,4-dihydroxy-n-(3-hydroxy-propyl)-3,3-dimethyl-butyramide
172. D-(+)-2,4-dihydroxy-3,3-dimethyl-n-(3-hydroxypropyl)butyramide
173. D-panthenol, >=98% (perchloric Acid Titration), >=98% (tlc)
174. Dexpanthenol, United States Pharmacopeia (usp) Reference Standard
175. (d)-(+)-2, 4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethylbutyramide
176. Butanamide, 2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethyl-, (theta)-
177. D(+)-.alpha.,.gamma.-dihydroxy-n-(3-hydroxypropyl)-.beta.,.beta.-dimethylbutyramide
178. Dexpanthenol, Pharmaceutical Secondary Standard; Certified Reference Material
179. 1113-70-8
| Molecular Weight | 205.25 g/mol |
|---|---|
| Molecular Formula | C9H19NO4 |
| XLogP3 | -0.9 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | 205.13140809 g/mol |
| Monoisotopic Mass | 205.13140809 g/mol |
| Topological Polar Surface Area | 89.8 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 182 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Dexpanthenol (topical) relieves itching and aids healing of skin in mild eczemas and dermatoses; itching skin, minor wounds, stings, bites, poison ivy, poison oak (dry sage) and minor skin irritations. Also, used in infants and children for diaper rash, chafing and mild skin irritations.
Novak, K.M. (ed.). Drug Facts and Comparisons2008 Edition. Wolters Kluwer Health. St. Louis, Missouri 2008., p. 2636
Prophylactic use immediately after major abdominal surgery to minimize the possibility of paralytic ileus. Intestinal atony causing abdominal distention; postoperative or post partum retention of flatus, or post operative delay in resumption of intestinal motility; paralytic ileus.
Novak, K.M. (ed.). Drug Facts and Comparisons2008 Edition. Wolters Kluwer Health. St. Louis, Missouri 2008., p. 1767
Vet: ... Dexpanthenol ... /is/ often used as source of B5. Only the D-isomers are active biologically, but dl-isomers are often used ... Equivalents: 1 g D-pantothenic acid = 936 mg D-dexpanthenol.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 645
/Exptl Use (Vet)/: The effect of B-complex vitamin on experimental liver damage in rats was studied. Ip injection of panthenol inhibited initial deposit of lipids after having removed 2/3 of the regenerating fatty liver in rats.
PMID:4235781 Petzold H, Weigel K; Int Z Vitaminforsch 38 (1): 97 (1968)
For more Therapeutic Uses (Complete) data for Dexpanthenol (13 total), please visit the HSDB record page.
Administration of dexpanthenol injection directly into the vein is not advised.
Novak, K.M. (ed.). Drug Facts and Comparisons2008 Edition. Wolters Kluwer Health. St. Louis, Missouri 2008., p. 1767
One case of heartburn and a few cases of GI cramps have been reported after dexpanthenol administration. Allergic reactions to dexpanthenol have been reported occasionally; however, these reactions have not been directly attributed to the drug. Although isolated reports of itching, tingling, difficulty in breathing, erythema, generalized dermatitis, urticaria, temporary respiratory difficulty (when dexpanthenol injection was administered 5 minutes after succinylcholine had been discontinued), hypotension, persistent (up to 10 days) diarrhea, and agitation have been associated with use of dexpanthenol injection, a causal relationship to the drug has not been established.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
It is not known whether dexpanthenol can cause fetal harm when administered to pregnant women. Dexpanthenol injection should be used during pregnancy only when clearly needed.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
Dexpanthenol injection should not be used for the management of mechanical obstruction; in these patients, therapy should be directed mainly at correcting the obstruction. The manufacturer of dexpanthenol injection cautions that the management of adynamic ileus includes correction of fluid and electrolyte abnormalities (especially hypokalemia), anemia, and hypoproteinemia; treatment of infection; avoidance of drugs that decrease GI motility; and decompression of the GI tract using nasogastric suction or a long intestinal tube when there is considerable distention.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
For more Drug Warnings (Complete) data for Dexpanthenol (9 total), please visit the HSDB record page.
Injection: Prophylactic use immediately after major abdominal surgery to minimize the possibility of paralytic ileus. Intestinal atony causing abdominal distention; postoperative or postpartum retention of flatus, or postoperative delay in resumption of intestinal motility; paralytic ileus. Topical: This medication is used as a moisturizer to treat or prevent dry, rough, scaly, itchy skin and minor skin irritations (e.g., diaper rash, skin burns from radiation therapy).
Pantothenic acid is a precursor of coenzyme A, which serves as a cofactor for a variety of enzyme-catalyzed reactions involving transfer of acetyl groups. The final step in the synthesis of acetylcholine consists of the choline acetylase transfer of acetyl group from acetylcoenzyme A to choline. Acetylcholine is the neurohumoral transmitter in the parasympathetic system and as such maintains the normal functions of the intestine. Decrease in acetylcholine content would result in decreased peristalsis and in extreme cases adynamic ileus.
A - Alimentary tract and metabolism
A11 - Vitamins
A11H - Other plain vitamin preparations
A11HA - Other plain vitamin preparations
A11HA30 - Dexpanthenol
D - Dermatologicals
D03 - Preparations for treatment of wounds and ulcers
D03A - Cicatrizants
D03AX - Other cicatrizants
D03AX03 - Dexpanthenol
S - Sensory organs
S01 - Ophthalmologicals
S01X - Other ophthalmologicals
S01XA - Other ophthalmologicals
S01XA12 - Dexpanthenol
Absorption
Dexpanthenol is soluble in water and alcohol, although insoluble in fats and oil based substances. With the appropriate vehicle, Dexpanthenol is easily penetrated into the skin. Rate of penetration and absorption is reduced when Dexpanthenol is administered as an oil/water formula.
Route of Elimination
Milk of nursing mothers receiving a normal diet contains about 2 ug of pantothenic acid per mL. About 70% of an oral dose of pantothenic acid is excreted unchanged in urine and about 30% in feces.
Volume of Distribution
Dexpanthenol is readily converted to pantothenic acid which is widely distributed into body tissues, mainly as coenzyme A. Highest concentrations are found in the liver, adrenal glands, heart, and kidneys.
Dexpanthenol is readily converted to pantothenic acid which is widely distributed into body tissues, mainly as coenzyme A. Highest concentrations are found in the liver, adrenal glands, heart, and kidneys. Milk of nursing mothers receiving a normal diet contains about 2 ug of pantothenic acid per mL. About 70% of an oral dose of pantothenic acid is excreted unchanged in urine and about 30% in feces.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
Dexpanthenol is readily converted to pantothenic acid which is widely distributed into body tissues, mainly as coenzyme A.
Dexpanthenol is readily converted to pantothenic acid which is widely distributed into body tissues, mainly as coenzyme A.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
Dexpanthenol is converted to pantothenic acid ... which then produces acetylcholine.
Evaluations of Drug Interactions. 1st ed. and supplements. Washington, DC: American Pharmaceutical Assn., 1973, 1974., p. 396
Half life have not been reported
Dexpanthenol is an alcohol derivative of pantothenic acid, a component of the B complex vitamins and an essential component of a normally functioning epithelium. Dexpanthenol is enzymatically cleaved to form pantothenic acid, which is an essential component of Coenzyme A, which acts as a cofactor in many enzymatic reactions that are important for protein metabolism in the epithelium. Dermatological effects of the topical use of dexpanthenol include increased fibroblast proliferation and accelerated re-epithelialization in wound healing. Furthermore, it acts as a topical protectant, moisturizer, and has demonstrated anti-inflammatory properties.
This alcohol ... is said to increase the amount of coenzyme A available for the synthesis of acetylcholine. Increased formation of acetylcholine is thought to increase peristalsis and intestinal tone.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 752
... To test the functional effect of pantothenate on dermal fibroblasts, cells were cultured and in vitro proliferation tests were performed using a standardized scratch test procedure. For all three donors analyzed, a strong stimulatory effect of pantothenate at a concentration of 20 ug/mL on the proliferation of cultivated dermal fibroblasts was observed. To study the molecular mechanisms resulting in the proliferative effect of pantothenate, gene expression was analyzed in dermal fibroblasts cultivated with 20 ug/mL of pantothenate compared with untreated cells using the GeneChip Human Exon 1.0 ST Array. A number of significantly regulated genes were identified including genes coding for interleukin (IL)-6, IL-8, Id1, HMOX-1, HspB7, CYP1B1 and MARCH-II. Regulation of these genes was subsequently verified by quantitative real-time polymerase chain reaction analysis. Induction of HMOX-1 expression by pantothenol and pantothenic acid in dermal cells was confirmed on the protein level using immunoblots. Functional studies revealed the enhanced suppression of free radical formation in skin fibroblasts cultured with panthenol. In conclusion, these studies provided new insight in the molecular mechanisms linked to the stimulatory effect of pantothenate and panthenol on the proliferation of dermal fibroblasts. /Calcium pantotenate/
Wiederholt T et al; Exper Dermatol 18 (11): 969-78 (2009). Available from, as of March 16, 2010: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=19397697
... Pantothenic acid, pantothenol and other derivatives ... are precursors of CoA /that/ protect cells and whole organs against peroxidative damage by increasing the content of cell glutathione...
Slyshenkov V et al; FEBS Lett 569 (1-3): 169-72 (2004). Available from, as of March 16, 2010: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15225628

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
36
PharmaCompass offers a list of D-Panthenol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right D-Panthenol manufacturer or D-Panthenol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred D-Panthenol manufacturer or D-Panthenol supplier.
PharmaCompass also assists you with knowing the D-Panthenol API Price utilized in the formulation of products. D-Panthenol API Price is not always fixed or binding as the D-Panthenol Price is obtained through a variety of data sources. The D-Panthenol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Dexpanthenol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Dexpanthenol, including repackagers and relabelers. The FDA regulates Dexpanthenol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Dexpanthenol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Dexpanthenol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Dexpanthenol supplier is an individual or a company that provides Dexpanthenol active pharmaceutical ingredient (API) or Dexpanthenol finished formulations upon request. The Dexpanthenol suppliers may include Dexpanthenol API manufacturers, exporters, distributors and traders.
click here to find a list of Dexpanthenol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Dexpanthenol DMF (Drug Master File) is a document detailing the whole manufacturing process of Dexpanthenol active pharmaceutical ingredient (API) in detail. Different forms of Dexpanthenol DMFs exist exist since differing nations have different regulations, such as Dexpanthenol USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Dexpanthenol DMF submitted to regulatory agencies in the US is known as a USDMF. Dexpanthenol USDMF includes data on Dexpanthenol's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Dexpanthenol USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Dexpanthenol suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Dexpanthenol Drug Master File in Japan (Dexpanthenol JDMF) empowers Dexpanthenol API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Dexpanthenol JDMF during the approval evaluation for pharmaceutical products. At the time of Dexpanthenol JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Dexpanthenol suppliers with JDMF on PharmaCompass.
A Dexpanthenol CEP of the European Pharmacopoeia monograph is often referred to as a Dexpanthenol Certificate of Suitability (COS). The purpose of a Dexpanthenol CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Dexpanthenol EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Dexpanthenol to their clients by showing that a Dexpanthenol CEP has been issued for it. The manufacturer submits a Dexpanthenol CEP (COS) as part of the market authorization procedure, and it takes on the role of a Dexpanthenol CEP holder for the record. Additionally, the data presented in the Dexpanthenol CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Dexpanthenol DMF.
A Dexpanthenol CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Dexpanthenol CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Dexpanthenol suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Dexpanthenol as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Dexpanthenol API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Dexpanthenol as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Dexpanthenol and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Dexpanthenol NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Dexpanthenol suppliers with NDC on PharmaCompass.
Dexpanthenol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Dexpanthenol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Dexpanthenol GMP manufacturer or Dexpanthenol GMP API supplier for your needs.
A Dexpanthenol CoA (Certificate of Analysis) is a formal document that attests to Dexpanthenol's compliance with Dexpanthenol specifications and serves as a tool for batch-level quality control.
Dexpanthenol CoA mostly includes findings from lab analyses of a specific batch. For each Dexpanthenol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Dexpanthenol may be tested according to a variety of international standards, such as European Pharmacopoeia (Dexpanthenol EP), Dexpanthenol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Dexpanthenol USP).
