
Synopsis
Synopsis
0
CEP/COS
0
KDMF
0
VMF
0
Australia

0
South Africa

0
Listed Dossiers
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
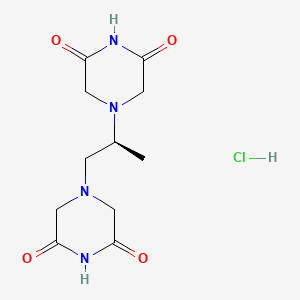

1. Adr 529
2. Adr-529
3. Adr529
4. Cardioxan
5. Cardioxane
6. Dexrazoxane
7. Hydrochloride, Dexrazoxane
8. Icrf 187
9. Icrf-187
10. Icrf187
11. Nsc 169780
12. Nsc-169780
13. Nsc169780
14. Razoxane, (s)-isomer
15. Razoxane, (s)-isomer, Hydrochloride
16. Zinecard
1. 149003-01-0
2. Dexrazoxane Hcl
3. Totect
4. Cardioxane
5. Zinecard
6. Cardioxan
7. Savene
8. Adr-529 Hydrochloride
9. Icrf-187 Hydrochloride
10. Icrf-187
11. 1263283-43-7
12. (+)-razoxane Hydrochloride
13. Razoxane Hydrochloride, (s)-
14. Dexrazoxane (hydrochloride)
15. 4-[(2s)-2-(3,5-dioxopiperazin-1-yl)propyl]piperazine-2,6-dione Hydrochloride
16. Dexrazoxane Hydrochloride [vandf]
17. Dexrazoxane Hydrochloride [who-dd]
18. 2,6-piperazinedione, 4,4'-((1s)-1-methyl-1,2-ethanediyl)bis-, Hydrochloride (1:1)
19. Razoxane (+)-form Hydrochloride [mi]
20. Dexrazoxane Hydrochloride [orange Book]
21. 5346058q7s
22. (s)-4,4'-(propane-1,2-diyl)bis(piperazine-2,6-dione) Hydrochloride
23. Dexrazoxane Hcl (icrf-187, Adr-529)
24. 2,6-piperazinedione, 4,4'-(1-methyl-1,2-ethanediyl)bis-, Hydrochloride, (s)-
25. Chebi:50224
26. Icrf 187 Hydrochloride
27. Topotect
28. Unii-5346058q7s
29. Zinecard (tn)
30. (s)-4,4'-(1-methyl-1,2-ethanediyl)bis-2,6-piperazinedione Hydrochloride
31. Savene (tn)
32. Totect (tn)
33. Dexrazoxanehydrochloride
34. Cardioxane Hydrochloride
35. Schembl18188
36. Chembl1200778
37. Dtxsid60164152
38. 4-[(2s)-2-(3,5-dioxopiperazin-1-yl)propyl]piperazine-2,6-dione;hydrochloride
39. Kdx-0811
40. S1222
41. Akos015900046
42. Ac-9014
43. Ccg-267515
44. As-16976
45. Dexrazoxane Hcl (icrf-187; Adr-529)
46. Sw220147-1
47. C72836
48. D07807
49. 003d010
50. Dexrazoxane Hydrochloride (icrf-187, Adr-529)
51. Q27121988
| Molecular Weight | 304.73 g/mol |
|---|---|
| Molecular Formula | C11H17ClN4O4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 304.0938327 g/mol |
| Monoisotopic Mass | 304.0938327 g/mol |
| Topological Polar Surface Area | 98.8 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 404 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 6 | |
|---|---|
| Drug Name | Dexrazoxane hydrochloride |
| PubMed Health | Dexrazoxane (Injection) |
| Drug Classes | Cardioprotective Agent, Dermatological Agent |
| Active Ingredient | Dexrazoxane hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500mg base/vial; eq 250mg base/vial |
| Market Status | Prescription |
| Company | Mylan Institutional; Eurohlth Intl |
| 2 of 6 | |
|---|---|
| Drug Name | Totect |
| Active Ingredient | Dexrazoxane hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500mg base/vial |
| Market Status | Prescription |
| Company | Biocodex |
| 3 of 6 | |
|---|---|
| Drug Name | Zinecard |
| PubMed Health | Dexrazoxane (Injection) |
| Drug Classes | Cardioprotective Agent, Dermatological Agent |
| Drug Label | ZINECARD (dexrazoxane for injection), a cardioprotective agent for use in conjunction with doxorubicin, is a sterile, pyrogen-free lyophilizate intended for intravenous administration.Chemically, dexrazoxane is (S)-4,4'-(1-methyl-1,2-ethanediyl)bis-2 |
| Active Ingredient | Dexrazoxane hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500mg base/vial; eq 250mg base/vial |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 4 of 6 | |
|---|---|
| Drug Name | Dexrazoxane hydrochloride |
| PubMed Health | Dexrazoxane (Injection) |
| Drug Classes | Cardioprotective Agent, Dermatological Agent |
| Active Ingredient | Dexrazoxane hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500mg base/vial; eq 250mg base/vial |
| Market Status | Prescription |
| Company | Mylan Institutional; Eurohlth Intl |
| 5 of 6 | |
|---|---|
| Drug Name | Totect |
| Active Ingredient | Dexrazoxane hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500mg base/vial |
| Market Status | Prescription |
| Company | Biocodex |
| 6 of 6 | |
|---|---|
| Drug Name | Zinecard |
| PubMed Health | Dexrazoxane (Injection) |
| Drug Classes | Cardioprotective Agent, Dermatological Agent |
| Drug Label | ZINECARD (dexrazoxane for injection), a cardioprotective agent for use in conjunction with doxorubicin, is a sterile, pyrogen-free lyophilizate intended for intravenous administration.Chemically, dexrazoxane is (S)-4,4'-(1-methyl-1,2-ethanediyl)bis-2 |
| Active Ingredient | Dexrazoxane hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500mg base/vial; eq 250mg base/vial |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
Savene is indicated for the treatment of anthracycline extravasation.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Cardiotonic Agents
Agents that have a strengthening effect on the heart or that can increase cardiac output. They may be CARDIAC GLYCOSIDES; SYMPATHOMIMETICS; or other drugs. They are used after MYOCARDIAL INFARCT; CARDIAC SURGICAL PROCEDURES; in SHOCK; or in congestive heart failure (HEART FAILURE). (See all compounds classified as Cardiotonic Agents.)
Topoisomerase II Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE II. Included in this category are a variety of ANTINEOPLASTIC AGENTS which target the eukaryotic form of topoisomerase II and ANTIBACTERIAL AGENTS which target the prokaryotic form of topoisomerase II. (See all compounds classified as Topoisomerase II Inhibitors.)
V03AF02
V03AF02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38186
Submission : 2023-03-17
Status : Active
Type : II
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 19002
Submission : 2005-12-05
Status : Active
Type : II

| Available Reg Filing : ASMF |

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 18336
Submission : 2005-05-09
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 23172
Submission : 2009-09-22
Status : Active
Type : II
Date of Issue : 2019-03-05
Valid Till : 2022-03-04
Written Confirmation Number : WC-0427
Address of the Firm :


GDUFA
DMF Review : Reviewed
Rev. Date : 2014-09-29
Pay. Date : 2014-02-14
DMF Number : 27826
Submission : 2014-02-04
Status : Active
Type : II
Date of Issue : 2017-07-05
Valid Till : 2020-07-05
Written Confirmation Number : WC-0399
Address of the Firm :


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 21542
Submission : 2008-04-17
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2020-10-08
Pay. Date : 2020-08-12
DMF Number : 34694
Submission : 2020-03-14
Status : Active
Type : II

Date of Issue : 2022-09-02
Valid Till : 2025-05-05
Written Confirmation Number : WC-0349
Address of the Firm :

NDC Package Code : 54893-0097
Start Marketing Date : 2020-03-13
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2022-12-12
Pay. Date : 2022-11-22
DMF Number : 37716
Submission : 2022-11-22
Status : Active
Type : II

NDC Package Code : 59651-735
Start Marketing Date : 2023-12-18
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (50kg/50kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15184
Submission : 2000-12-07
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38186
Submission : 2023-03-17
Status : Active
Type : II
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 19002
Submission : 2005-12-05
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 18336
Submission : 2005-05-09
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2022-12-12
Pay. Date : 2022-11-22
DMF Number : 37716
Submission : 2022-11-22
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 21542
Submission : 2008-04-17
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15184
Submission : 2000-12-07
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2014-09-29
Pay. Date : 2014-02-14
DMF Number : 27826
Submission : 2014-02-04
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 23172
Submission : 2009-09-22
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2020-10-08
Pay. Date : 2020-08-12
DMF Number : 34694
Submission : 2020-03-14
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Registration Number : 224MF10205
Registrant's Address : Piazzale Luigi Cadorna, 4 - 20123 MILANO, Italy
Initial Date of Registration : 2012-10-09
Latest Date of Registration : 2021-01-20

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Date of Issue : 2019-03-05
Valid Till : 2022-03-04
Written Confirmation Number : WC-0427
Address of the Firm : Plot No 49 & 50, J.N Pharma City, Parawada (M), Visakhapatanam -531019, Andhra P...

Date of Issue : 2017-07-05
Valid Till : 2020-07-05
Written Confirmation Number : WC-0399
Address of the Firm : Sy. No. 143 to 148 150 and 151 Near Gandimaisamma Cross Roads, D.P Pally, Dundig...

Date of Issue : 2022-09-02
Valid Till : 2025-05-05
Written Confirmation Number : WC-0349
Address of the Firm : MIs. MSN Laboratories Private Limited, Unit-II, sv. No, 50, Kardanur (Village), ...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]NDC Package Code : 59651-735
Start Marketing Date : 2023-12-18
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (50kg/50kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 54893-0097
Start Marketing Date : 2020-03-13
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 50384-0200
Start Marketing Date : 2005-05-09
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (450g/450g)
Marketing Category : BULK INGREDIENT

NDC Package Code : 33656-0001
Start Marketing Date : 2010-11-29
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]About the Company : Transo-Pharm, a fully licensed and certified distributor, specializes in pharmaceutical components for the health and veterinary industries. It offers support to clients throughout...
About the Company : Porton Pharma Solutions Ltd. was founded in 2005, & the company's stock was successfully listed in Shenzhen Stock Exchange in 2014. Our R&D, manufacturing & operation facilities ar...
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
About the Company : Founded in 1935, TAPI Technology & API Services has a long-standing tradition of advancing health through innovation and dedication. Today, we proudly build upon this legacy, drivi...
About the Company : A globally renowned manufacturer of Small Volume Parenterals (SVPs), Gland Pharma was founded in 1978 at Hyderabad by a visionary, PVN Raju, who has always thought far ahead of his...

About the Company : Established in 2002, Tecoland represents selected cGMP manufacturers with proven capabilities in organic synthesis, fermentation production as well as process and method developmen...

About the Company : Yangtze River Pharmaceutical Group (hereinafter refers to YRPG) was founded in 1971, with over 9000 employees. Comprehensive competitiveness of YRPG has been ranked in the top five...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Under the terms of the divestment, Clinigen will divest four medicines, Cardioxane (dexrazoxane), Savene, Totect and Ethyol, all of which help mitigate the side effects patients may experience when treated with other cancer therapies, to CNX Therapeutics.
Lead Product(s): Dexrazoxane
Therapeutic Area: Oncology Brand Name: Totect
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: CNX Therapeutics
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Divestment October 08, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Dexrazoxane
Therapeutic Area : Oncology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : CNX Therapeutics
Deal Size : Undisclosed
Deal Type : Divestment
Clinigen Divests Global Rights to Four Cancer Support Therapies to CNX Therapeutics
Details : Under the terms of the divestment, Clinigen will divest four medicines, Cardioxane (dexrazoxane), Savene, Totect and Ethyol, all of which help mitigate the side effects patients may experience when treated with other cancer therapies, to CNX Therapeutics...
Product Name : Totect
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
October 08, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Totect® now can be used for reducing the incidence and severity of cardiomyopathy associated with doxorubicin in women with breast cancer who have received a cumulative doxorubicin dose of 300 mg/m² and who will continue to receive doxorubicin therapy.
Lead Product(s): Dexrazoxane
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Totect
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable October 11, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Dexrazoxane
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Clinigen’s Totect® Receives FDA Approval for New Indication to Treat Incidence and Severity of ...
Details : Totect® now can be used for reducing the incidence and severity of cardiomyopathy associated with doxorubicin in women with breast cancer who have received a cumulative doxorubicin dose of 300 mg/m² and who will continue to receive doxorubicin therapy.
Product Name : Totect
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
October 11, 2020

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : DEXRAZOXANE HYDROCHLORIDE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 500MG BASE/VIAL
Packaging :
Approval Date : 2011-10-19
Application Number : 200752
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : TOTECT
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 500MG BASE/VIAL **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Packaging :
Approval Date : 2007-09-06
Application Number : 22025
Regulatory Info : DISCN
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Savene
Dosage Form : POWDER FOR CONCENTRATE AND SOLVENT
Dosage Strength : 20 MG / ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : Cardioxane
Dosage Form : Dexrazoxano 500Mg 1 Unit Parenteral Use
Dosage Strength : 1 ampoule EV 500 mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Savene
Dosage Form : Powder to concentrate and liquid to the infusion fluid, resolution
Dosage Strength : 20 mg/ml
Packaging : Set
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : DEXRAZOXANE HYDROCHLORIDE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 500MG BASE/VIAL
Packaging :
Approval Date : 2016-11-28
Application Number : 207321
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : DEXRAZOXANE HYDROCHLORIDE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 250MG BASE/VIAL
Packaging :
Approval Date : 2019-12-16
Application Number : 207321
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : DEXRAZOXANE HYDROCHLORIDE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 250MG BASE/VIAL
Packaging :
Approval Date : 2004-09-28
Application Number : 76068
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : DEXRAZOXANE HYDROCHLORIDE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 500MG BASE/VIAL
Packaging :
Approval Date : 2004-09-28
Application Number : 76068
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : ZINECARD
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 500MG BASE/VIAL
Packaging :
Approval Date : 1995-05-26
Application Number : 20212
Regulatory Info : DISCN
Registration Country : USA

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
34
PharmaCompass offers a list of Dexrazoxane API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Dexrazoxane manufacturer or Dexrazoxane supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Dexrazoxane manufacturer or Dexrazoxane supplier.
PharmaCompass also assists you with knowing the Dexrazoxane API Price utilized in the formulation of products. Dexrazoxane API Price is not always fixed or binding as the Dexrazoxane Price is obtained through a variety of data sources. The Dexrazoxane Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Dexrazoxane manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Dexrazoxane, including repackagers and relabelers. The FDA regulates Dexrazoxane manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Dexrazoxane API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Dexrazoxane manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Dexrazoxane supplier is an individual or a company that provides Dexrazoxane active pharmaceutical ingredient (API) or Dexrazoxane finished formulations upon request. The Dexrazoxane suppliers may include Dexrazoxane API manufacturers, exporters, distributors and traders.
click here to find a list of Dexrazoxane suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Dexrazoxane DMF (Drug Master File) is a document detailing the whole manufacturing process of Dexrazoxane active pharmaceutical ingredient (API) in detail. Different forms of Dexrazoxane DMFs exist exist since differing nations have different regulations, such as Dexrazoxane USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Dexrazoxane DMF submitted to regulatory agencies in the US is known as a USDMF. Dexrazoxane USDMF includes data on Dexrazoxane's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Dexrazoxane USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Dexrazoxane suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Dexrazoxane Drug Master File in Japan (Dexrazoxane JDMF) empowers Dexrazoxane API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Dexrazoxane JDMF during the approval evaluation for pharmaceutical products. At the time of Dexrazoxane JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Dexrazoxane suppliers with JDMF on PharmaCompass.
A Dexrazoxane written confirmation (Dexrazoxane WC) is an official document issued by a regulatory agency to a Dexrazoxane manufacturer, verifying that the manufacturing facility of a Dexrazoxane active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Dexrazoxane APIs or Dexrazoxane finished pharmaceutical products to another nation, regulatory agencies frequently require a Dexrazoxane WC (written confirmation) as part of the regulatory process.
click here to find a list of Dexrazoxane suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Dexrazoxane as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Dexrazoxane API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Dexrazoxane as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Dexrazoxane and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Dexrazoxane NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Dexrazoxane suppliers with NDC on PharmaCompass.
Dexrazoxane Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Dexrazoxane GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Dexrazoxane GMP manufacturer or Dexrazoxane GMP API supplier for your needs.
A Dexrazoxane CoA (Certificate of Analysis) is a formal document that attests to Dexrazoxane's compliance with Dexrazoxane specifications and serves as a tool for batch-level quality control.
Dexrazoxane CoA mostly includes findings from lab analyses of a specific batch. For each Dexrazoxane CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Dexrazoxane may be tested according to a variety of international standards, such as European Pharmacopoeia (Dexrazoxane EP), Dexrazoxane JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Dexrazoxane USP).
