
Synopsis
Synopsis
0
CEP/COS
0
KDMF
0
VMF
0
Australia

0
South Africa

0
Listed Dossiers
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Adr 529
2. Adr-529
3. Adr529
4. Cardioxan
5. Cardioxane
6. Dexrazoxane
7. Hydrochloride, Dexrazoxane
8. Icrf 187
9. Icrf-187
10. Icrf187
11. Nsc 169780
12. Nsc-169780
13. Nsc169780
14. Razoxane, (s)-isomer
15. Razoxane, (s)-isomer, Hydrochloride
16. Zinecard
1. 149003-01-0
2. Dexrazoxane Hcl
3. Totect
4. Cardioxane
5. Zinecard
6. Cardioxan
7. Savene
8. Adr-529 Hydrochloride
9. Icrf-187 Hydrochloride
10. Icrf-187
11. 1263283-43-7
12. (+)-razoxane Hydrochloride
13. Razoxane Hydrochloride, (s)-
14. Dexrazoxane (hydrochloride)
15. 4-[(2s)-2-(3,5-dioxopiperazin-1-yl)propyl]piperazine-2,6-dione Hydrochloride
16. Dexrazoxane Hydrochloride [vandf]
17. Dexrazoxane Hydrochloride [who-dd]
18. 2,6-piperazinedione, 4,4'-((1s)-1-methyl-1,2-ethanediyl)bis-, Hydrochloride (1:1)
19. Razoxane (+)-form Hydrochloride [mi]
20. Dexrazoxane Hydrochloride [orange Book]
21. 5346058q7s
22. (s)-4,4'-(propane-1,2-diyl)bis(piperazine-2,6-dione) Hydrochloride
23. Dexrazoxane Hcl (icrf-187, Adr-529)
24. 2,6-piperazinedione, 4,4'-(1-methyl-1,2-ethanediyl)bis-, Hydrochloride, (s)-
25. Chebi:50224
26. Icrf 187 Hydrochloride
27. Topotect
28. Unii-5346058q7s
29. Zinecard (tn)
30. (s)-4,4'-(1-methyl-1,2-ethanediyl)bis-2,6-piperazinedione Hydrochloride
31. Savene (tn)
32. Totect (tn)
33. Dexrazoxanehydrochloride
34. Cardioxane Hydrochloride
35. Schembl18188
36. Chembl1200778
37. Dtxsid60164152
38. 4-[(2s)-2-(3,5-dioxopiperazin-1-yl)propyl]piperazine-2,6-dione;hydrochloride
39. Kdx-0811
40. S1222
41. Akos015900046
42. Ac-9014
43. Ccg-267515
44. As-16976
45. Dexrazoxane Hcl (icrf-187; Adr-529)
46. Sw220147-1
47. C72836
48. D07807
49. 003d010
50. Dexrazoxane Hydrochloride (icrf-187, Adr-529)
51. Q27121988
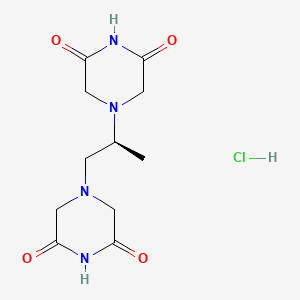
| Molecular Weight | 304.73 g/mol |
|---|---|
| Molecular Formula | C11H17ClN4O4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 304.0938327 g/mol |
| Monoisotopic Mass | 304.0938327 g/mol |
| Topological Polar Surface Area | 98.8 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 404 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 6 | |
|---|---|
| Drug Name | Dexrazoxane hydrochloride |
| PubMed Health | Dexrazoxane (Injection) |
| Drug Classes | Cardioprotective Agent, Dermatological Agent |
| Active Ingredient | Dexrazoxane hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500mg base/vial; eq 250mg base/vial |
| Market Status | Prescription |
| Company | Mylan Institutional; Eurohlth Intl |
| 2 of 6 | |
|---|---|
| Drug Name | Totect |
| Active Ingredient | Dexrazoxane hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500mg base/vial |
| Market Status | Prescription |
| Company | Biocodex |
| 3 of 6 | |
|---|---|
| Drug Name | Zinecard |
| PubMed Health | Dexrazoxane (Injection) |
| Drug Classes | Cardioprotective Agent, Dermatological Agent |
| Drug Label | ZINECARD (dexrazoxane for injection), a cardioprotective agent for use in conjunction with doxorubicin, is a sterile, pyrogen-free lyophilizate intended for intravenous administration.Chemically, dexrazoxane is (S)-4,4'-(1-methyl-1,2-ethanediyl)bis-2 |
| Active Ingredient | Dexrazoxane hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500mg base/vial; eq 250mg base/vial |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 4 of 6 | |
|---|---|
| Drug Name | Dexrazoxane hydrochloride |
| PubMed Health | Dexrazoxane (Injection) |
| Drug Classes | Cardioprotective Agent, Dermatological Agent |
| Active Ingredient | Dexrazoxane hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500mg base/vial; eq 250mg base/vial |
| Market Status | Prescription |
| Company | Mylan Institutional; Eurohlth Intl |
| 5 of 6 | |
|---|---|
| Drug Name | Totect |
| Active Ingredient | Dexrazoxane hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500mg base/vial |
| Market Status | Prescription |
| Company | Biocodex |
| 6 of 6 | |
|---|---|
| Drug Name | Zinecard |
| PubMed Health | Dexrazoxane (Injection) |
| Drug Classes | Cardioprotective Agent, Dermatological Agent |
| Drug Label | ZINECARD (dexrazoxane for injection), a cardioprotective agent for use in conjunction with doxorubicin, is a sterile, pyrogen-free lyophilizate intended for intravenous administration.Chemically, dexrazoxane is (S)-4,4'-(1-methyl-1,2-ethanediyl)bis-2 |
| Active Ingredient | Dexrazoxane hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500mg base/vial; eq 250mg base/vial |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
Savene is indicated for the treatment of anthracycline extravasation.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Cardiotonic Agents
Agents that have a strengthening effect on the heart or that can increase cardiac output. They may be CARDIAC GLYCOSIDES; SYMPATHOMIMETICS; or other drugs. They are used after MYOCARDIAL INFARCT; CARDIAC SURGICAL PROCEDURES; in SHOCK; or in congestive heart failure (HEART FAILURE). (See all compounds classified as Cardiotonic Agents.)
Topoisomerase II Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE II. Included in this category are a variety of ANTINEOPLASTIC AGENTS which target the eukaryotic form of topoisomerase II and ANTIBACTERIAL AGENTS which target the prokaryotic form of topoisomerase II. (See all compounds classified as Topoisomerase II Inhibitors.)
V03AF02
V03AF02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38186
Submission : 2023-03-17
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 23172
Submission : 2009-09-22
Status : Active
Type : II
Date of Issue : 2019-03-05
Valid Till : 2022-03-04
Written Confirmation Number : WC-0427
Address of the Firm :


GDUFA
DMF Review : Reviewed
Rev. Date : 2014-09-29
Pay. Date : 2014-02-14
DMF Number : 27826
Submission : 2014-02-04
Status : Active
Type : II
Date of Issue : 2017-07-05
Valid Till : 2020-07-05
Written Confirmation Number : WC-0399
Address of the Firm :


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 19002
Submission : 2005-12-05
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2020-10-08
Pay. Date : 2020-08-12
DMF Number : 34694
Submission : 2020-03-14
Status : Active
Type : II

Date of Issue : 2022-09-02
Valid Till : 2025-05-05
Written Confirmation Number : WC-0349
Address of the Firm :

NDC Package Code : 54893-0097
Start Marketing Date : 2020-03-13
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 18336
Submission : 2005-05-09
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2022-12-12
Pay. Date : 2022-11-22
DMF Number : 37716
Submission : 2022-11-22
Status : Active
Type : II

NDC Package Code : 59651-735
Start Marketing Date : 2023-12-18
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (50kg/50kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 21542
Submission : 2008-04-17
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15184
Submission : 2000-12-07
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?