
Synopsis
0
CEP/COS
0
KDMF
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Regulatory FDF Prices
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
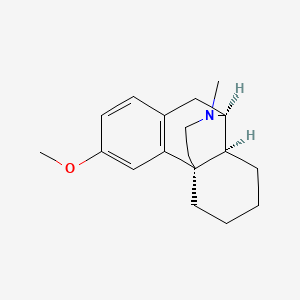

1. D-methorphan
2. Delsym
3. Dextromethorphan Hydrobromide
4. Dextromethorphan Hydrobromide, (+-)-isomer
5. Dextromethorphan Hydrobromide, Monohydrate
6. Dextromethorphan Hydrochloride
7. Dextromethorphan, (+-)-isomer
8. Hydrobromide, Dextromethorphan
9. Hydrochloride, Dextromethorphan
10. L-methorphan
11. Levomethorphan
12. Racemethorphan
1. D-methorphan
2. 125-71-3
3. Delta-methorphan
4. Dextromorphan
5. Dextromethorfan
6. Destrometerfano
7. Dextrometorfano
8. Albutussin
9. Dextromethorphane
10. Dextromethorphanum
11. Ba 2666
12. (+)-dextromethorphan
13. Benylin Dm
14. Nodex
15. Hsdb 3056
16. Dextromethorfan [czech]
17. Destrometerfano [dcit]
18. Morphinan, 3-methoxy-17-methyl-, (9alpha,13alpha,14alpha)-
19. 3-methoxy-17-methyl-9alpha,13alpha,14alpha-morphinan
20. Dextrometorphan
21. Romilar
22. Dextrometorfano [inn-spanish]
23. (+)-3-methoxy-17-methylmorphinan
24. 7355x3rots
25. Nsc-751452
26. (1s,9s,10s)-4-methoxy-17-methyl-17-azatetracyclo[7.5.3.01,10.02,7]heptadeca-2(7),3,5-triene
27. Ba-2666
28. Morphinan, 3-methoxy-17-methyl-, (9.alpha.,13.alpha.,14.alpha.)-
29. Balminil Dm
30. 3-methoxy-17-methyl-9.alpha.,13.alpha.,14.alpha.-morphinan
31. Dextromethorphane [inn-french]
32. Dextromethorphanum [inn-latin]
33. Dextromethorphan [usp:inn:ban]
34. Dxm [antitussive]
35. ( )-3-methoxy-n-methylmorphinon
36. Dextromethorphan (usp)
37. Einecs 204-752-2
38. Chebi:4470
39. Brn 0088549
40. Unii-7355x3rots
41. Calmylin
42. (+)-3-methoxy-n-methylmorphinon
43. Delsym (salt/mix)
44. Medicon (salt/mix)
45. Romilar (salt/mix)
46. Tusilan (salt/mix)
47. 9alpha,13alpha,14alpha-morphinan, 3-methoxy-17-methyl-
48. Prestwick0_000359
49. Prestwick1_000359
50. Prestwick2_000359
51. Prestwick3_000359
52. Lopac-d-2531
53. Racemethorphan [as D-form]
54. ( )-cis-1,3,4,9,10,10a-hexahydro-6-methoxy-11-methyl-2h-10,4alpha-iminoethanophenanthren
55. Schembl29949
56. Bspbio_000457
57. Dextromethorphan [mi]
58. 4-21-00-01367 (beilstein Handbook Reference)
59. Chembl52440
60. Dextromethorphan [inn]
61. Spbio_002378
62. Dextromethorphan [hsdb]
63. Bpbio1_000503
64. Gtpl6953
65. Dextromethorphan [vandf]
66. (9alpha,13alpha,14alpha)-3-methoxy-17-methylmorphinan
67. Dextromethorphan [mart.]
68. Dtxsid3022908
69. Chebi:92579
70. (+)-3-methoxy-n-methylmorphinan
71. Dextromethorphan [usp-rs]
72. Dextromethorphan [who-dd]
73. (9alpha,13alpha,14alpha)-17-methyl-3-(methyloxy)morphinan
74. 9-alpha,13-alpha,14-alpha-morphinan, 3-methoxy-17-methyl-
75. Hms2090c08
76. Lsm-2726
77. Morphinan, 3-methoxy-17-methyl-, (9-alpha,13-alpha,14-alpha)-
78. Zinc3201907
79. Bdbm50366613
80. Akos025311415
81. Db00514
82. Nsc 751452
83. (+)-cis-1,3,4,9,10,10a-hexahydro-6-methoxy-11-methyl-2h-10,4alpha-iminoethanophenanthren
84. Dextromethorphan [usp Monograph]
85. Ncgc00015333-01
86. Ncgc00015333-02
87. 3-methoxy-17-methyl-9a,13a,14a-morphinan
88. (9a,13a,14a)-3-methoxy-17-methylmorphinan
89. C06947
90. D03742
91. Q407781
92. 3-methoxy-17-methyl-9.alpha.,13.alpha.-morphinan
93. J-005274
94. Brd-k33211335-337-03-7
95. Morphinan, 3-methoxy-17-methyl-, (9?,13?,14?)-
96. (+)-3-methoxy-17-methyl-9alpha,13alpha,14alpha-morphinan
97. 9.alpha.,13.alpha.,14.alpha.-morphinan, 3-methoxy-17-methyl-
98. 9alpha,13alpha,14alpha-morphinan, 3-methoxy-17-methyl- (8ci)
99. 3-methoxy-17-methylmorphinan-, (9.alpha.,13.alpha.,14.alpha.)- #
100. 4-methoxy-12-methyl-12-azatetracyclo[9.3^1.10^.0^2.7^] Heptadeca-2(7),3,5-triene
101. (1r,9r,10r)-4-methoxy-17-methyl-17-azatetracyclo[7.5.3.0^{1,10}.0^{2,7}]heptadeca-2,4,6-triene
102. (4as,10s,10as)-6-methoxy-11-methyl-1,3,4,9,10,10a-hexahydro-2h-10,4a-(epiminoethano)phenanthrene
103. Dextromethorphan Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 271.4 g/mol |
|---|---|
| Molecular Formula | C18H25NO |
| XLogP3 | 3.4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 271.193614421 g/mol |
| Monoisotopic Mass | 271.193614421 g/mol |
| Topological Polar Surface Area | 12.5 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 370 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Delsym |
| Active Ingredient | Dextromethorphan polistirex |
| Dosage Form | Suspension, extended release |
| Route | Oral |
| Strength | eq 30mg hbr/5ml |
| Market Status | Over the Counter |
| Company | Reckitt Benckiser |
| 2 of 2 | |
|---|---|
| Drug Name | Delsym |
| Active Ingredient | Dextromethorphan polistirex |
| Dosage Form | Suspension, extended release |
| Route | Oral |
| Strength | eq 30mg hbr/5ml |
| Market Status | Over the Counter |
| Company | Reckitt Benckiser |
Antitussive Agents; Excitatory Amino Acid Antagonists
National Library of Medicine's Medical Subject Headings. Dextromethorphan. Online file (MeSH, 2017). Available from, as of August 30, 2017: https://www.nlm.nih.gov/mesh/2017/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Dextromethorphan is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of August 30, 2017: https://clinicaltrials.gov/
Dextromethorphan is used for the temporary relief of coughs caused by minor throat and bronchial irritation such as may occur with common colds or with inhaled irritants. Dextromethorphan is most effective in the treatment of chronic, nonproductive cough. The drug is a common ingredient in commercial cough mixtures available without prescription.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2931
Dextromethorphan preparations are administered orally. Lozenges containing dextromethorphan hydrobromide should not be used in children younger than 6 years of age and liquid-filled capsules containing the drug should not be used in children younger than 12 years of age, unless otherwise directed by a clinician.
OLSON, K.R. (Ed). Poisoning and Drug Overdose, Sixth Edition. McGraw-Hill, New York, NY 2012, p. 2931
For more Therapeutic Uses (Complete) data for Dextromethorphan (20 total), please visit the HSDB record page.
Administration of dextromethorphan may be associated with histamine release, and the drug should be used with caution in atopic children. Dextromethorphan also should be used with caution in sedated or debilitated patients and in patients confined to the supine position. Dextromethorphan should not be taken for persistent or chronic cough (e.g., with smoking, emphysema, asthma) or when coughing is accompanied by excessive secretions, unless directed by a clinician. If cough persists for longer than 1 week, tends to recur, or is accompanied by high fever, rash, or persistent headache, a clinician should be consulted.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2931
Individuals with phenylketonuria (i.e., homozygous deficiency of phenylalanine hydroxylase) and other individuals who must restrict their intake of phenylalanine should be warned that some commercially available preparations of dextromethorphan contain aspartame, which is metabolized in the GI tract to phenylalanine following oral administration.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2931
Adverse effects with dextromethorphan are rare, but nausea and/or other GI disturbances, slight drowsiness, and dizziness sometimes occur. The drug produces no analgesia or addiction and little or no CNS depression.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2931
Although cough and cold preparations that contain cough suppressants (including dextromethorphan), nasal decongestants, antihistamines, and/or expectorants commonly are used in pediatric patients younger than 2 years of age, systematic reviews of controlled trials have concluded that nonprescription (over-the-counter, OTC) cough and cold preparations are not more effective than placebo in reducing acute cough and other symptoms of upper respiratory tract infection in these patients. Furthermore, adverse events, including deaths, have been (and continue to be) reported in pediatric patients younger than 2 years of age receiving these preparations.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2931
For more Drug Warnings (Complete) data for Dextromethorphan (7 total), please visit the HSDB record page.
Dextromethorphan is indicated in combination with [brompheniramine] and [pseudoephedrine] in the treatment of coughs and upper respiratory symptoms associated with allergies or the common cold. Dextromethorphan is also used in combination with [guaifenesin] as an over-the-counter product to relieve a cough. Dextromethorphan in combination with [quinidine] is indicated in the treatment of pseudobulbar affect.
Dextromethorphan is an opioid-like molecule indicated in combination with other medication in the treatment of coughs and pseudobulbar affect. It has a moderate therapeutic window, as intoxication can occur at higher doses. Dextromethorphan has a moderate duration of action. Patients should be counselled regarding the risk of intoxication.
Antitussive Agents
Agents that suppress cough. They act centrally on the medullary cough center. EXPECTORANTS, also used in the treatment of cough, act locally. (See all compounds classified as Antitussive Agents.)
Excitatory Amino Acid Antagonists
Drugs that bind to but do not activate excitatory amino acid receptors, thereby blocking the actions of agonists. (See all compounds classified as Excitatory Amino Acid Antagonists.)
R05DA09
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
R - Respiratory system
R05 - Cough and cold preparations
R05D - Cough suppressants, excl. combinations with expectorants
R05DA - Opium alkaloids and derivatives
R05DA09 - Dextromethorphan
Absorption
A 30mg oral dose of dextromethorphan reaches a Cmax of 2.9 ng/mL, with a Tmax of 2.86 h, and an AUC of 17.8 ng\*h/mL.
Volume of Distribution
The volume of distribution of dextromethorphan is 5-6.7L/kg.
Dextromethorphan is rapidly absorbed from the GI tract and exerts its antitussive effect in 15-30 minutes after oral administration. The duration of action is approximately 3-6 hours with conventional dosage forms.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2932
Dextromethorphan and its metabolites are excreted via the kidney. Depending on the metabolism phenotype up to 11% may be excreted unchanged or up to 100% as demethylated conjugated morphinan compounds. In the first 24 hours after dosing, less than 0.1% is eliminated in the feces.
International Programme on Chemical Safety; Poisons Information Monograph: Dextromethorphan (PIM 179) (1997). Available from, as of August 30, 2017: https://www.inchem.org/pages/pims.html
Dextromethorphan is well absorbed from the gastrointestinal tract with maximum serum level occurring at 2.5 hours. Peak concentration of the major metabolite dextrorphan) was 1.6 to 1.7 hours.
International Programme on Chemical Safety; Poisons Information Monograph: Dextromethorphan (PIM 179) (1997). Available from, as of August 30, 2017: https://www.inchem.org/pages/pims.html
Dextromethorphan hydrobromide (DM) is a widely used antitussive. This study determined, for the first time, the basic pharmacokinetic profile of DM and its active metabolite, dextrorphan (DP) in children and adolescents. Thirty-eight male and female subjects at risk for developing an upper respiratory tract infection (URTI), or symptomatic with cough due to URTI, were enrolled in this single-dose, open-label study: ages 2-5 years (Group A, n = 8), 6-11 years (Group B, n = 17), 12-17 years (Group C, n = 13). Subjects were genotyped for cytochrome P450 (CYP) 2D6 polymorphisms and characterized as poor (PM) or non-poor metabolizers (non-PM). Groups A and B were dosed using an age-weight dosing schedule (DM range 7.5-24.75 mg); a 30-mg dose was used for Group C. Average exposures to total DP increased as age group increased, and average exposure to DM was highest in the adolescent group. One subject in that group was a PM. The terminal half-life values were longer in the adolescent group due in part to the single PM subject. No relationship between body weight and pharmacokinetic parameters was noted. This is the first evaluation of the pharmacokinetic characteristics of DM in children and adolescents. A single dose of DM in this population was safe, and well tolerated at all doses tested. The data are used to model and compare pediatric DM exposures with those of adults.
PMID:25027615 Guenin E et al; Clin Drug Investig 34 (9): 609-16 (2014)
For more Absorption, Distribution and Excretion (Complete) data for Dextromethorphan (7 total), please visit the HSDB record page.
Dextromethorphan can be N-demethylated to 3-methoxymorphinan by CYP3A4, CYP2D6, and CYP2C9 or O-demethylated to dextrorphan by CYP2D6 and CYP2C9. Dextrorphan is N-demethylated by CYP3A4 and CYP2D6, while 3-methoxymorphinan is O-demethylated by CYP2D6. Both are metabolized to form 3-hydroxymorphinan. Dextrorphan and 3-hydroxymorphinan are both O-glucuronidated or O-sulfated.
Genetic polymorphism has profound effects on its metabolism. Dextromethorphan undergoes polymorphic metabolism depending on variation in cytochrome P-450 enzyme phenotype. The specific cytochrome P-450 enzyme is P450 2D6(CYP2D6). Fast metabolizers constitute about 84% of the population. After a 30 mg dose plasma levels are less than 5 ng/mL four hours postingestion. Intermediate metabolizers constitute about 6.8% of the population. After an oral dose of 30 mg plasma levels are 10 to 20 ng/mL at 4 hours and less than 5 ng/mL at 24 hours postingestion. Poor metabolizers constitute 5% to 10% of the Caucasian population. The ratio of metabolite to parent drug in 8 hour urine sample is less than 10 to 1 after a 15 mg dose. After an oral dose of 30 mg plasma levels are greater than 10 ng/mL at 4 hours and greater than 5 ng/mL at 24 hours.
International Programme on Chemical Safety; Poisons Information Monograph: Dextromethorphan (PIM 179) (1997). Available from, as of August 30, 2017: https://www.inchem.org/pages/pims.html
There is a clear first pass metabolism and it is generally assumed that the therapeutic activity is primarily due to its active metabolite, dextrophan.
International Programme on Chemical Safety; Poisons Information Monograph: Dextromethorphan (PIM 179) (1997). Available from, as of August 30, 2017: https://www.inchem.org/pages/pims.html
It is metabolized in the liver by extensive metabolizers to dextrorphan. Dextrorphan is itself an active antitussive compound. Only small amounts are formed in poor metabolizers. Less than 15% of the dose form minor metabolites including D-methoxymorphinane.
International Programme on Chemical Safety; Poisons Information Monograph: Dextromethorphan (PIM 179) (1997). Available from, as of August 30, 2017: https://www.inchem.org/pages/pims.html
The pentose phosphate pathway (PPP) is involved in the activity of glucose-6-phosphate dehydrogenase (G6PD) and generation of NADPH, which plays a key role in drug metabolism. The aim of this study was to investigate the effects of modulation of the PPP on drug metabolism capacity in vitro. A pair of hepatic cell lines, ie, the cancerous HepG2 cells and normal L02 cells, was used. The expression of CYP450 enzymes, p53 and G6PD in the cells were analyzed. The metabolism of testosterone (TEST, 10 umol/L) and dextromethorphan (DEM, 1 umol/L), the two typical substrates for CYP3A4 and CYP2D6, in the cells was examined in the presence of different agents. Both the expression and metabolic activities of CYP3A4 and CYP2D6 were considerably higher in HepG2 cells than in L02 cells. The metabolism of TEST and DEM in HepG2 cells was dose-dependently inhibited by the specific CYP3A4 inhibitor ketoconazole and CYP2D6 inhibitor quinidine. Addition of the p53 inhibitor cyclic PFT-alpha (5, 25 umol/L) in HepG2 cells dose-dependently enhanced the metabolism of DEM and TEST, whereas addition of the p53 activator NSC 66811 (3, 10, 25 umol/L) dose-dependently inhibited the metabolism. Furthermore, addition of the G6PD inhibitor 6-aminonicotinamide (5, 15 umol/L) in HepG2 cells dose-dependently inhibited the metabolism of DEM and TEST, whereas addition of the PPP activity stimulator menadione (1, 5, 15 umol/L) dose-dependently enhanced the metabolism. Modulation of p53 and the PPP alters the metabolism of DEM and TEST, suggesting that the metabolic flux pattern of PPP may be closely involved in drug metabolism and the individual variance.
PMID:25619394 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4326794 Xiao WJ et al; Acta Pharmacol Sin 36 (2): 259-67 (2015)
Dextromethorphan has known human metabolites that include 3-methoxymorphinan and Dextrorphan.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Dextromethorphan has a half life of 3-30 hours.
The half life of /dextromethorphan/ is approximately 2 to 4 hours in people with normal metabolism.
International Programme on Chemical Safety; Poisons Information Monograph: Dextromethorphan (PIM 179) (1997). Available from, as of August 30, 2017: https://www.inchem.org/pages/pims.html
Dextromethorphan is an agonist of NMDA and sigma-1 receptors. It is also an antagonist of 3/4 nicotinic receptors. However, the mechanism by which dextromethorphan's receptor agonism and antagonism translates to a clinical effect is not well understood.
Dextromethorphan (DXM) is the dextro isomer of levomethorphan, a semisynthetic morphine derivative. Although structurally similar to other /CNS depressants/, DXM does not act as a mu receptor opioid (eg, morphine, heroin). DXM and its metabolite, dextrorphan, act as potent blockers of the N-methyl-d-aspartate (NMDA) receptor.
Department of Justice; Drug Enforcement Administration (DEA), Dextromethorphan (March 2014). Available from, as of August 30, 2017: https://www.deadiversion.usdoj.gov/drug_chem_info/dextro_m.pdf
Amantadine and dextromethorphan suppress levodopa (L-DOPA)-induced dyskinesia (LID) in patients with Parkinson's disease (PD) and abnormal involuntary movements (AIMs) in the unilateral 6-hydroxydopamine (6-OHDA) rat model. These effects have been attributed to N-methyl-d-aspartate (NMDA) antagonism. However, amantadine and dextromethorphan are also thought to block serotonin (5-HT) uptake and cause 5-HT overflow, leading to stimulation of 5-HT(1A) receptors, which has been shown to reduce LID. We undertook a study in 6-OHDA rats to determine whether the anti-dyskinetic effects of these two compounds are mediated by NMDA antagonism and/or 5-HT(1A) agonism. In addition, we assessed the sensorimotor effects of these drugs using the Vibrissae-Stimulated Forelimb Placement and Cylinder tests. Our data show that the AIM-suppressing effect of amantadine was not affected by the 5-HT(1A) antagonist WAY-100635, but was partially reversed by the NMDA agonist d-cycloserine. Conversely, the AIM-suppressing effect of dextromethorphan was prevented by WAY-100635 but not by d-cycloserine. Neither amantadine nor dextromethorphan affected the therapeutic effects of L-DOPA in sensorimotor tests. We conclude that the anti-dyskinetic effect of amantadine is partially dependent on NMDA antagonism, while dextromethorphan suppresses AIMs via indirect 5-HT(1A) agonism. Combined with previous work from our group, our results support the investigation of 5-HT(1A) agonists as pharmacotherapies for LID in PD patients.
PMID:22861201 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3573705 Paquette MA et al; Eur J Neurosci 36 (9): 3224-34 (2012)
Dextromethorphan (DM) is a dextrorotatory morphinan and an over-the-counter non-opioid cough suppressant. We have previously shown that DM protects against LPS-induced dopaminergic neurodegeneration through inhibition of microglia activation. Here, we investigated protective effects of DM against endotoxin shock induced by lipopolysaccharide/d-galactosamine (LPS/GalN) in mice and the mechanism underlying its protective effect. Mice were given multiple injections of DM (12.5 mg/kg, s.c.) 30 min before and 2, 4 hr after an injection of LPS/GalN (20 ug/700 mg/kg). DM administration decreased LPS/GalN-induced mortality and hepatotoxicity, as evidenced by increased survival rate, decreased serum alanine aminotransferase activity and improved pathology. Furthermore, DM was also effective when it was given 30 min after LPS/GalN injection. The protection was likely associated with reduced serum and liver tumor necrosis factor alpha (TNF-alpha) levels. DM also attenuated production of superoxide and intracellular reactive oxygen species in Kupffer cells and neutrophils. Real-time RT-PCR analysis revealed that DM administration suppressed the expression of a variety of inflammation-related genes such as macrophage inflammatory protein-2, CXC chemokine, thrombospondin-1, intercellular adhesion molecular-1 and interleukin-6. DM also decreased the expression of genes related to cell-death pathways, such as the DNA damage protein genes GADD45 and GADD153. In summary, DM is effective in protecting mice against LPS/GalN-induced hepatotoxicity, and the mechanism is likely through a faster TNF-alpha clearance, and decrease of superoxide production and inflammation and cell-death related components. This study not only extends neuroprotective effect of DM, but also suggests that DM may be a novel compound for the therapeutic intervention for sepsis.
PMID:15627475 Li G et al; Biochem Pharmacol 69 (2): 233-40 (2005)
/The investigators/ showed that dextromethorphan (DM) provides neuroprotective/anticonvulsant effects and that DM and its major metabolite, dextrorphan /DX/, have a high-affinity for sigma(1) receptors, but a low affinity for sigma(2) receptors. In addition, we found that DM has a higher affinity than DX for sigma(1) sites, whereas DX has a higher affinity than DM for PCP sites. We extend our earlier findings by showing that DM attenuated trimethyltin (TMT)-induced neurotoxicity (convulsions, hippocampal degeneration and spatial memory impairment) in rats. This attenuation was reversed by the sigma(1) receptor antagonist BD 1047, but not by the sigma(2) receptor antagonist ifenprodil. DM attenuates TMT-induced reduction in the sigma(1) receptor-like immunoreactivity of the rat hippocampus, this attenuation was blocked by the treatment with BD 1047, but not by ifenprodil. These results suggest that DM prevents TMT-induced neurotoxicity, at least in part, via sigma(1) receptor stimulation.
PMID:17386960 Shin EJ et al; Neurochem Int 50 (6): 791-9 (2007)
Dextromethorphan (DEX) is a widely used non-opioid antitussive. However, the precise site of action and its mechanism were not fully understood. We examined the effects of DEX on AMPA receptor-mediated glutamatergic transmission in the nucleus tractus solitarius (NTS) of guinea pigs. Excitatory postsynaptic currents (evoked EPSCs: eEPSCs) were evoked in the second-order neurons by electrical stimulation of the tractus solitarius. DEX reversibly decreased the eEPSC amplitude in a concentration-dependent manner. The DEX-induced inhibition of eEPSC was accompanied by an increased paired-pulse ratio. Miniature EPSCs (mEPSCs) were also recorded in the presence of Cd(2+) or tetrodotoxin. DEX decreased the frequency of mEPSCs without affecting their amplitude. Topically applied AMPA provoked an inward current in the neurons, which was unchanged during the perfusion of DEX. BD1047, a sigma-1-receptor antagonist, did not block the inhibitory effect of DEX on the eEPSCs, but antagonized the inhibition of eEPSCs induced by SKF-10047, a sigma-1 agonist. Haloperidol, a sigma-1 and -2 receptor ligand, had no influence on the inhibitory action of DEX. These results suggest that DEX inhibits glutamate release from the presynaptic terminals projecting to the second-order NTS neurons, but this effect of DEX is not mediated by the activation of sigma receptors.
PMID:21487194 Ohi Y et al; J Pharmacol Sci 116 (1): 54-62 (2011)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Company : Acella Pharmace
Brompheniramine/Pseudoephed/DM
Drug Cost (USD) : 3,780
Year : 2023
Prescribers : 137
Prescriptions : 146

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Eywa Pharma Inc
Brompheniramine/Pseudoephed/DM
Drug Cost (USD) : 1,349
Year : 2023
Prescribers : 51
Prescriptions : 59

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Perrigo/Padagis
Brompheniramine/Pseudoephed/DM
Drug Cost (USD) : 2,047
Year : 2023
Prescribers : 85
Prescriptions : 92

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Pharm Assoc Inc
Brompheniramine/Pseudoephed/DM
Drug Cost (USD) : 929
Year : 2023
Prescribers : 32
Prescriptions : 35

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Amneal Pharmace
Promethazine/Dextromethorphan
Drug Cost (USD) : 39,521
Year : 2023
Prescribers : 2576
Prescriptions : 3233

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Morton Grove Ph
Promethazine/Dextromethorphan
Drug Cost (USD) : 12,450
Year : 2023
Prescribers : 897
Prescriptions : 1019

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Slate Run Pharm
Promethazine/Dextromethorphan
Drug Cost (USD) : 55,397
Year : 2023
Prescribers : 3315
Prescriptions : 4365

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Tris Pharma Inc
Promethazine/Dextromethorphan
Drug Cost (USD) : 12,388
Year : 2023
Prescribers : 772
Prescriptions : 1005

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Wockhardt USA L
Promethazine/Dextromethorphan
Drug Cost (USD) : 14,837
Year : 2023
Prescribers : 942
Prescriptions : 1132

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Acella Pharmace
Brompheniramine/Pseudoephed/DM
Drug Cost (USD) : 3,644
Year : 2022
Prescribers : 128
Prescriptions : 150

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Reply
13 Dec 2025
Reply
04 Nov 2025
Reply
23 Jan 2025
Reply
16 Dec 2024
Reply
07 Sep 2023
Reply
21 Oct 2022
Reply
02 Apr 2021
Reply
20 Jul 2020
Reply
22 Jan 2020
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
REF. STANDARDS & IMPURITIES

 Sigma-Aldrich empowers scientific discovery with top-quality solutions to accelerate research, innovation, and better health worldwide.
Sigma-Aldrich empowers scientific discovery with top-quality solutions to accelerate research, innovation, and better health worldwide.
CAS Number :
Quantity Per Vial :
Sale Unit :
Price : $775.00
Details :
Monograph : PHR2206-100MG
Storage : +2°C to +30°C
Code/Batch No :
 Sigma-Aldrich empowers scientific discovery with top-quality solutions to accelerate research, innovation, and better health worldwide.
Sigma-Aldrich empowers scientific discovery with top-quality solutions to accelerate research, innovation, and better health worldwide.
CAS Number :
Quantity Per Vial :
Sale Unit :
Price : $1,400.00
Details :
Monograph : PHR1976-30MG
Storage : -10°C to -25°C
Code/Batch No :
 Sigma-Aldrich empowers scientific discovery with top-quality solutions to accelerate research, innovation, and better health worldwide.
Sigma-Aldrich empowers scientific discovery with top-quality solutions to accelerate research, innovation, and better health worldwide.
CAS Number : 6700-34-1
Quantity Per Vial :
Sale Unit :
Price : $125.00
Details :
Monograph : PHR1018-500MG
Storage : +2°C to +30°C
Code/Batch No :
 Sigma-Aldrich empowers scientific discovery with top-quality solutions to accelerate research, innovation, and better health worldwide.
Sigma-Aldrich empowers scientific discovery with top-quality solutions to accelerate research, innovation, and better health worldwide.
CAS Number : 6700-34-1
Quantity Per Vial :
Sale Unit :
Price : $566.00
Details :
Monograph : PHR1018-10G
Storage : +2°C to +30°C
Code/Batch No :
Dextromethorphan EP Impurity A
CAS Number : 1531-23-3
Quantity Per Vial :
Sale Unit :
Price :
Details : In stock
Monograph :
Storage :
Code/Batch No : D0053.03

CAS Number : 57969-05-8
Quantity Per Vial :
Sale Unit :
Price :
Details : In stock
Monograph :
Storage :
Code/Batch No : D0053.04

CAS Number :
Quantity Per Vial :
Sale Unit :
Price :
Details : In stock
Monograph :
Storage :
Code/Batch No : D0053.05

CAS Number :
Quantity Per Vial :
Sale Unit :
Price : $566.00
Details :
Monograph : PHR1018-10G
Storage : +2?C to +30?C
Code/Batch No :

CAS Number :
Quantity Per Vial :
Sale Unit :
Price : $125.00
Details :
Monograph : PHR1018-500MG
Storage : +2?C to +30?C
Code/Batch No :

CAS Number :
Quantity Per Vial :
Sale Unit :
Price : $1,400.00
Details :
Monograph : PHR1976-30MG
Storage : -10?C to -25?C
Code/Batch No :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE
72
PharmaCompass offers a list of Dextromethorphan API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Dextromethorphan manufacturer or Dextromethorphan supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Dextromethorphan manufacturer or Dextromethorphan supplier.
PharmaCompass also assists you with knowing the Dextromethorphan API Price utilized in the formulation of products. Dextromethorphan API Price is not always fixed or binding as the Dextromethorphan Price is obtained through a variety of data sources. The Dextromethorphan Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Dextromethorphan manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Dextromethorphan, including repackagers and relabelers. The FDA regulates Dextromethorphan manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Dextromethorphan API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Dextromethorphan manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Dextromethorphan supplier is an individual or a company that provides Dextromethorphan active pharmaceutical ingredient (API) or Dextromethorphan finished formulations upon request. The Dextromethorphan suppliers may include Dextromethorphan API manufacturers, exporters, distributors and traders.
click here to find a list of Dextromethorphan suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Dextromethorphan DMF (Drug Master File) is a document detailing the whole manufacturing process of Dextromethorphan active pharmaceutical ingredient (API) in detail. Different forms of Dextromethorphan DMFs exist exist since differing nations have different regulations, such as Dextromethorphan USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Dextromethorphan DMF submitted to regulatory agencies in the US is known as a USDMF. Dextromethorphan USDMF includes data on Dextromethorphan's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Dextromethorphan USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Dextromethorphan suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Dextromethorphan Drug Master File in Japan (Dextromethorphan JDMF) empowers Dextromethorphan API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Dextromethorphan JDMF during the approval evaluation for pharmaceutical products. At the time of Dextromethorphan JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Dextromethorphan suppliers with JDMF on PharmaCompass.
A Dextromethorphan written confirmation (Dextromethorphan WC) is an official document issued by a regulatory agency to a Dextromethorphan manufacturer, verifying that the manufacturing facility of a Dextromethorphan active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Dextromethorphan APIs or Dextromethorphan finished pharmaceutical products to another nation, regulatory agencies frequently require a Dextromethorphan WC (written confirmation) as part of the regulatory process.
click here to find a list of Dextromethorphan suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Dextromethorphan as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Dextromethorphan API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Dextromethorphan as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Dextromethorphan and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Dextromethorphan NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Dextromethorphan suppliers with NDC on PharmaCompass.
Dextromethorphan Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Dextromethorphan GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Dextromethorphan GMP manufacturer or Dextromethorphan GMP API supplier for your needs.
A Dextromethorphan CoA (Certificate of Analysis) is a formal document that attests to Dextromethorphan's compliance with Dextromethorphan specifications and serves as a tool for batch-level quality control.
Dextromethorphan CoA mostly includes findings from lab analyses of a specific batch. For each Dextromethorphan CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Dextromethorphan may be tested according to a variety of international standards, such as European Pharmacopoeia (Dextromethorphan EP), Dextromethorphan JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Dextromethorphan USP).

