
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Canada

0
Australia

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
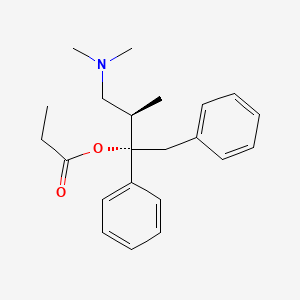

1. D Propoxyphene
2. D-propoxyphene
3. Darvon
4. Dextropropoxyphene
5. Hydrochloride, Propoxyphene
6. Propoxyphene Hydrochloride, (r*,r*)-(+-)-isomer
7. Propoxyphene Hydrochloride, (r-(r*,r*))-isomer
8. Propoxyphene Hydrochloride, (r-(r*,s*))-isomer
9. Propoxyphene Hydrochloride, (s-(r*,r*))-isomer
10. Propoxyphene Maleate, (+)-isomer
11. Propoxyphene Phosphate, (s-(r*,s*))-isomer
12. Propoxyphene Sulfate, (s-(r*,s*))-isomer
1. Dextropropoxyphene
2. D-propoxyphene
3. Dextropropoxyphen
4. Algafan
5. Dextroproxifeno
6. Destropropossifene
7. Antalvic
8. 469-62-5
9. Depromic
10. Proxagesic
11. Femadol
12. Dextropropoxifeno
13. Propoxyphene, (+)-
14. Dextropropoxyphenum
15. Sk 65
16. Propoxyphene, D-
17. 4-dimethylamino-3-methyl-1,2-diphenyl-2-propoxybutane
18. (+)-1,2-diphenyl-2-propionoxy-3-methyl-4-dimethylaminobutane
19. (+)-4-dimethylamino-1,2-diphenyl-3-methyl-2-propionyloxybutane
20. Alpha-(+)-4-dimethylamino-1,2-diphenyl-3-methyl-2-butanol Propionate Ester
21. (s)-alpha-(2-(dimethylamino)-1-methylethyl)-alpha-phenylbenzeneethanol Propanoate
22. Alpha-d-4-dimethylamino-3-methyl-1,2-diphenyl-2-butanol-propionat
23. Ii2g62ov6f
24. S2f83w92tk
25. Chebi:51173
26. Sk-65
27. (1s,2r)-1-benzyl-3-(dimethylamino)-2-methyl-1-phenylpropyl Propanoate
28. 2-butanol, 4-(dimethylamino)-3-methyl-1,2-diphenyl-, Propionate (ester), (2s,3r)-
29. J5.928e
30. Dextropropoxyphene (inn)
31. [(2s,3r)-4-(dimethylamino)-3-methyl-1,2-diphenylbutan-2-yl] Propanoate
32. 2-butanol, 4-(dimethylamino)-3-methyl-1,2-diphenyl-, Propionate, (+)-
33. Dextroproxifeno [spanish]
34. Propoxypene
35. Destropropossifene [dcit]
36. Dextropropoxyphene [inn]
37. Alpha-4-dimethylamino-1,2-diphenyl-3-methyl-2-butanol Propionate
38. Proxyvon
39. Dextropropoxifeno [inn-spanish]
40. Dextropropoxyphenum [inn-latin]
41. (d)-propoxyphene
42. Dextropropoxyphene [inn:ban]
43. Hsdb 3175
44. Einecs 207-420-5
45. Unii-ii2g62ov6f
46. Unii-s2f83w92tk
47. Usaf El-84
48. Bulk Dextropropoxyphene (non-dosage Forms)
49. Propoxyphene-d
50. Dea No. 9273
51. Alpha-d-4-dimethylamino-3-methyl-1,2-diphenyl-2-butanol Propionate
52. Propoxyphene, Dl-
53. Alpha-d-4-dimethylamino-3-methyl-1,2-diphenyl-2-butanol-propionat [german]
54. Darvon (salt/mix)
55. Dolene (salt/mix)
56. Abalgin (salt/mix)
57. Doloxen (salt/mix)
58. Erantin (salt/mix)
59. Romidon (salt/mix)
60. Benzeneethanol, Alpha-(2-(dimethylamino)-1-methylethyl)-alpha-phenyl-, Propanoate (ester), (s-(r*,s*))-
61. Doloxene (salt/mix)
62. C07406
63. D07809
64. .alpha.-d-propoxyphene
65. (+)-alpha-propoxyphene
66. Propoxyphene [mi]
67. (+)-propoxyphene
68. Alpha-(+)-4-dimethylamino-1,2-diphenyl-3-methyl-2-propionoxybutane
69. Propoxyphene [hsdb]
70. Propoxyphene [vandf]
71. Schembl25405
72. Methanol, Compd. With (+-)-alpha-(2-dimethylamino)-1-methylethyl-alpha- Phenylphenethyl Propionate (1:1)
73. (+/-)-propoxyphene
74. Gtpl7593
75. Chembl1213351
76. Dtxsid1023524
77. Bdbm82269
78. Propoxyphene, (+/-)-
79. Ids-nd-004(sect.2)
80. Dextropropoxyphene [mart.]
81. Dextropropoxyphene [who-dd]
82. Benzeneethanol, .alpha.-[2-(dimethylamino)-1-methylethyl]-.alpha.-phenyl-, Propanoate (ester), [s-(r*,s*)]-
83. Zinc1530769
84. Db00647
85. 2621-61-6
86. Benzeneethanol, Alpha-((1r)-2-(dimethylamino)-1-methylethyl)-alpha-phenyl-, Propanoate (ester), (alphas)-
87. Cas_469-62-5
88. Dextropropoxyphene 0.1 Mg/ml In Acetonitrile
89. Dextropropoxyphene 1.0 Mg/ml In Acetonitrile
90. Q2268608
91. (+)-4-(dimethylamino)-3-methyl-1,2-diphenyl-2-butanol Propionate
92. [(2s,3r)-4-dimethylamino-3-methyl-1,2-di(phenyl)butan-2-yl] Propanoate
93. (2s,3r)-(+)-4-(dimethylamino)-3-methyl-1,2-diphenyl-2-butanol Propionate (ester)
94. (2s,3r)-(+)-4-(dimethylamino)-3-methyl-1,2-diphenyl-2-butanol Propionate(ester)
95. .alpha.-(+)-4-dimethylamino-1,2-diphenyl-3-methyl-2-butanol Propionate Ester
96. 1-benzyl-3-(dimethylamino)-2-methyl-1-phenylpropyl Propionate, [s-(r*,s*)]- #
97. (+)-propoxyphene Solution, 1.0 Mg/ml In Acetonitrile, Ampule Of 1 Ml, Certified Reference Material
98. Benzeneethanol, .alpha.-((1r)-2-(dimethylamino)-1-methylethyl)-.alpha.-phenyl-, 1-propanoate, (.alpha.s)-
99. Benzeneethanol, .alpha.-((1r)-2-(dimethylamino)-1-methylethyl)-.alpha.-phenyl-, 1-propanoate, (.alpha.s)-rel-
100. Benzeneethanol, .alpha.-[2-(dimethylamino)-1-methylethyl]-.alpha.-phenyl-, Propanoate, [s-(r*,s*)]-
| Molecular Weight | 339.5 g/mol |
|---|---|
| Molecular Formula | C22H29NO2 |
| XLogP3 | 4.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 9 |
| Exact Mass | 339.219829168 g/mol |
| Monoisotopic Mass | 339.219829168 g/mol |
| Topological Polar Surface Area | 29.5 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 397 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Analgesics, Opioid; Antitussive Agents; Narcotics
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
The U.S. Food and Drug Administration (FDA) is recommending against continued prescribing and use of the pain reliever propoxyphene because new data show that the drug can cause serious toxicity to the heart, even when used at therapeutic doses. FDA has requested that companies voluntarily withdraw propoxyphene from the United States market. Propoxyphene is an opioid pain reliever used to treat mild to moderate pain. It is sold under various names as a single-ingredient product (e.g., Darvon) and as part of a combination product with acetaminophen (e.g., Darvocet). FDA's recommendation is based on all available data including data from a new study that evaluated the effects that increasing doses of propoxyphene have on the heart. The results of the new study showed that when propoxyphene was taken at therapeutic doses, there were significant changes to the electrical activity of the heart: prolonged PR interval, widened QRS complex and prolonged QT interval. These changes, which can be seen on an electrocardiogram (ECG), can increase the risk for serious abnormal heart rhythms. FDA has concluded that the safety risks of propoxyphene outweigh its benefits for pain relief at recommended doses. ... Additional Information for Healthcare Professionals: FDA recommends that healthcare professionals: Stop prescribing and dispensing propoxyphene-containing products to patients. Contact patients currently taking propoxyphene-containing products and ask them to discontinue the drug. Inform patients of the risks associated with propoxyphene. Discuss alternative pain management strategies other than propoxyphene with your patients. Be aware of the possible risk of cardiac conduction abnormalities (prolonged QT, PR, and QRS intervals) in patients taking propoxyphene and assess patients for these events if they present with any signs or symptoms of arrhythmia. Report any side effects with propoxyphene to FDA's MedWatch program.
US FDA; Drug Safety and Availability; FDA Drug Safety Communication: FDA Recommends Against the Continued Use of Propoxyphene (November 19, 2010). Available from, as of November 30, 2010: https://www.fda.gov/Drugs/DrugSafety/ucm234338.htm
The U.S. Food and Drug Administration is asking manufacturers of prescription combination products that contain acetaminophen to limit the amount of acetaminophen to no more than 325 milligrams (mg) in each tablet or capsule. The FDA also is requiring manufacturers to update labels of all prescription combination acetaminophen products to warn of the potential risk for severe liver injury. /Combination products containing acetaminophen/
US FDA; News and Events; Press Announcement: FDA Limits Acetaminophen in Prescription Combination Products; Requires Liver Toxicity Warnings; (January 13, 2011). Available from, as of March 27, 2011: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm239894.htm
Xanodyne Pharmaceuticals Inc. which makes Darvon and Darvocet, the brand version of the prescription pain medication propoxyphene, has agreed to withdraw the medication from the U.S. market at the request of the U.S. Food and Drug Administration. The FDA has also informed the generic manufacturers of propoxyphene-containing products of Xanodyne's decision and requested that they voluntarily remove their products as well. The FDA sought market withdrawal of propoxyphene after receiving new clinical data showing that the drug puts patients at risk of potentially serious or even fatal heart rhythm abnormalities. As a result of these data, combined with other information, including new epidemiological data, the agency concluded that the risks of the medication outweigh the benefits.
US FDA; Drug Safety and Availability; FDA Drug Safety Communication: Xanodyne Agrees to Withdraw Propoxyphene From the U.S. Market (November 19, 2010). Available from, as of March 30, 2011:: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2010/ucm234350.htm
For more Therapeutic Uses (Complete) data for (d)-PROPOXYPHENE (6 total), please visit the HSDB record page.
One should be alert for evidence of inadequate propoxyphene analgesia in smokers (especially heavy smokers); selection of an alternative analgesic may be appropriate in such patients.
Hansten P.D. Drug Interactions. 5th ed. Philadelphia: Lea and Febiger, 1985., p. 420
Propoxyphene should be admin with caution to patients who are dependent on opiates. Because propoxyphene will not support morphine dependence, the sudden substitution of the usual dosage of propoxyphene for opiates may result in acute opiate withdrawal symptoms; these symptoms may be avoided by gradually reducing the dosage of the prior medication as propoxyphene is substituted.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2196
Tolerance, psychologic dependence, and physical dependence have been reported in patients receiving propoxyphene. The dependence liability is qualitatively similar to that of codeine, but quantitatively less.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2196
Propoxyphene has been willfully abused by dissolving propoxyphene pellets formerly contained in the Darvon Compound preparations in water and injecting the solution IV. Inadvertent injection into the brachial artery has resulted in digital gangrene necessitating amputation. Abuse by oral ingestion of large doses of propoxyphene hydrochloride has also been reported.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2196
For more Drug Warnings (Complete) data for (d)-PROPOXYPHENE (13 total), please visit the HSDB record page.
Toxic doses for adults are stated to be 800 mg of the hydrochloride salt and 1200 mg of the napsylate salt. /Propoxyphene hydrochloride and napsylate/
Gossel, T.A., J.D. Bricker. Principles of Clinical Toxicology. 3rd ed. New York, NY: Raven Press, Ltd., 1994., p. 301
Toxic propoxyphene blood concentration: 30-60 ug/dL; Lethal propoxyphene blood concentration: 80-200 ug/dL /From table/
Gossel, T.A., J.D. Bricker. Principles of Clinical Toxicology. 3rd ed. New York, NY: Raven Press, Ltd., 1994., p. 298
For the relief of mild to moderate pain.
Propoxyphene, a synthetic opiate agonist, is structurally similar to methadone. Its general pharmacologic properties are those of the opiates as a group. The analgesic effect of propoxyphene is due to the d-isomer, dextropropoxyphene. It binds to the opiate receptors and leads to a decrease of the perception of pain stimuli. Propoxyphene possesses little to no antitussive activity and no antipyretic action.
Analgesics, Opioid
Compounds with activity like OPIATE ALKALOIDS, acting at OPIOID RECEPTORS. Properties include induction of ANALGESIA or NARCOSIS. (See all compounds classified as Analgesics, Opioid.)
Narcotics
Agents that induce NARCOSIS. Narcotics include agents that cause somnolence or induced sleep (STUPOR); natural or synthetic derivatives of OPIUM or MORPHINE or any substance that has such effects. They are potent inducers of ANALGESIA and OPIOID-RELATED DISORDERS. (See all compounds classified as Narcotics.)
N - Nervous system
N02 - Analgesics
N02A - Opioids
N02AC - Diphenylpropylamine derivatives
N02AC04 - Dextropropoxyphene
Route of Elimination
The major route of metabolism is cytochrome CYP3A4 mediated N-demethylation to norpropoxyphene, which is excreted by the kidneys. In 48 hours, approximately 20% to 25% of the administered dose of propoxyphene is excreted via the urine, most of which is free or conjugated norpropoxyphene.
Volume of Distribution
16 L/kg
Clearance
2.6 L/min
Following oral administration, propoxyphene hydrochloride and napsylate are absorbed principally in the upper small intestine. The napsylate salt appears to be absorbed more gradually than the hydrochloride salt. Equimolar doses of propoxyphene hydrochloride or napsylate provide similar plasma concentrations. The bioavailability of oral propoxyphene hydrochloride doses of 65, 130, or 195 mg is equivalent to that of oral propoxyphene napsylate doses of 100, 200, or 300 mg, respectively.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2197
Peak plasma propoxyphene concentrations are usually achieved within 2-2.5 hours following oral administration of propoxyphene hydrochloride capsules or propoxyphene napsylate suspension (no longer commercially available in the US) and within 3 hours following oral administration of propoxyphene napsylate tablets. The analgesic effect occurs within 15 minutes to 1 hour and persists for 4-6 hours. Therapeutic plasma propoxyphene concentrations are 50 ng/mL or greater. Peak plasma concentrations achieved with the recommended 65-mg dose of propoxyphene hydrochloride may range from 50-120 ng/mL, while concentrations of the norpropoxyphene metabolite range from 100-200 ng/mL.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2197
Only a small fraction of the absorbed dose (30%-70%) enters the general circulation in unmetabolized form. The rest is metabolized by intestinal and hepatic enzymes during absorption. Single dose kinetics indicate complete oral absorption. Peak plasma levels of propoxyphene after an oral 65 mg dose are 84-94 ng/ml (0.084-0.094 ug/ml) and are reached in 1.1-1.5 hr.
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 726
The delay in absorption has been attributed to slower gastric emptying and/or the physical barrier produced by the food mass. The effect of meal composition on the absorption of propoxyphene /Darvon/ has been studied in 6 healthy volunteers. On an empty stomach, peak plasma levels occurred at about 2 hr, while ... high carbohydrate & high fat meals delayed peak times to about 3 hr & high protein to about 4 hr. Although the absorption rate was decreased by food, high carbohydrate & high protein meals slightly increased the overall bioavailability of propoxyphene.
Hansten P.D. Drug Interactions. 5th ed. Philadelphia: Lea and Febiger, 1985., p. 419
For more Absorption, Distribution and Excretion (Complete) data for (d)-PROPOXYPHENE (11 total), please visit the HSDB record page.
Hepatic
Propoxyphene undergoes extensive first-pass metabolism by intestinal and hepatic enzymes. Propoxyphene is metabolized mainly via N-demethylation (mediated by cytochrome P-450 (CYP) isoenzyme 3A4) to form norpropoxyphene. Ring hydroxylation and glucuronide formation appear to be minor metabolic pathways for the drug. Norpropoxyphene has an elimination half-life of 30-36 hours. Norpropoxyphene and unchanged propoxyphene are excreted mainly in urine. Approximately 20-25% of an orally administered 65-mg dose of propoxyphene hydrochloride may be recovered in urine as unchanged drug (trace amount) and free or conjugated norpropoxyphene within 48 hours.It appears that the unchanged drug is excreted mainly within 6 hours and the metabolite is excreted in the 6- to 48-hour period following administration. Renal clearance of propoxyphene is about 2.6 L/minute.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2197
Formation of cytochrome p450 metabolic intermediate complexes in vivo occurred with propoxyphene in native & phenobarbital-induced rats. In vivo formation correlated with relative ability for it to form metabolic intermediate complexes & inhibit mixed function oxidation reactions in vitro.
PMID:491835 ROBERTS SM, FRANKLIN MR; LIFE SCI 25 (10): 845-51 (1979)
Yields 3-dimethylamino-1,2-diphenyl-2-butyl propionate-n-oxide iN pig & 3-methylamino-1,2-diphenyl-2-butyl propionate iN rat. From table/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. D-83
Presystemic metabolism is believed to occur mainly in the liver with some minor intestinal participation. The aim of this study was to investigate the respective part of each of these two organs in the metabolism of the analgesic d-propoxyphene. Pharmacological doses of d-propoxyphene were given in the duodenum (ID), the portal vein (IP), and the femoral vein (IV) of male Wistar rats. A tracer dose of (14)C-d-propoxyphene was also administered either in IV, IP, or ID as well as in hepatectomized rats or rats with bile duct diversion. In vitro demethylation occurring in liver and intestinal microsomes was also studied. Absolute DP bioavailability obtained after oral administration was two times higher than that observed after portal administration (48.9% vs. 23.2%, respectively), an result opposite (i.e. a lower bioavailability) of that expected on the basis of the existence of a liver enzyme saturation phenomenon. The (14)C-d-propoxyphene cumulative excretion after (14)C-d-propoxyphene administration was significantly lower after IV or ID administration than after injection in the portal vein as a bolus or within 20 min. The biliary excretion of the labeled compound varied in the opposite direction, being greater after IV or ID than after IP administration, suggesting that the metabolism of d-propoxyphene in the liver is influenced by an extrahepatic transformation. This most likely occurs in the gut since the production of (14)C-d-propoxyphene after IV administration was similar to that after ID administration. This transformation did not prohibit d-propoxyphene detection in the systemic blood but was sufficient to increase the part eliminated with bile and to decrease the part demethylated NP. Demethylation mainly occurs in the liver since the production of (14)C-d-propoxyphene was nearly abolished in hepatectomized rats. Furthermore, microsomes of hepatic but not of intestinal origin were able to demethylate d-propoxyphene. Our data suggest that the transformation of d-propoxyphene occurring in gut after oral administration is responsible for changes in the hepatic metabolism of the drug.
PMID:9351901 Horsmans Y et al; Drug Metab Dispos 25 (11): 1257-9 (1997)
Dextropropoxyphene has known human metabolites that include Nordextropropoxyphene.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
6-12 hours
Propoxyphene has an elimination half-life of 6-12 hours.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2197
The half life of elimination of the parent compound is 6 to 12 hours. The half life of elimination of norpropoxyphene is 30 to 36 hours.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 773
... Norpropoxyphene has a longer plasma half-life in the dog than propoxyphene. /Propoxyphene hydrochloride/
Booth, N.H., L.E. McDonald (eds.). Veterinary Pharmacology and Therapeutics. 5th ed. Ames, Iowa: Iowa State University Press, 1982., p. 291
In geriatric patients 70-78 years of age, elimination half-lives of propoxyphene and norpropoxyphene reportedly were 13-35 and 22-41 hours, respectively.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2198
Propoxyphene acts as a weak agonist at OP1, OP2, and OP3 opiate receptors within the central nervous system (CNS). Propoxyphene primarily affects OP3 receptors, which are coupled with G-protein receptors and function as modulators, both positive and negative, of synaptic transmission via G-proteins that activate effector proteins. Binding of the opiate stimulates the exchange of GTP for GDP on the G-protein complex. As the effector system is adenylate cyclase and cAMP located at the inner surface of the plasma membrane, opioids decrease intracellular cAMP by inhibiting adenylate cyclase. Subsequently, the release of nociceptive neurotransmitters such as substance P, GABA, dopamine, acetylcholine, and noradrenaline is inhibited. Opioids such as propoxyphene also inhibit the release of vasopressin, somatostatin, insulin, and glucagon. Opioids close N-type voltage-operated calcium channels (OP2-receptor agonist) and open calcium-dependent inwardly rectifying potassium channels (OP3 and OP1 receptor agonist). This results in hyperpolarization and reduced neuronal excitability.
... Propoxyphene binds primarily to mu-opioid receptors & produces analgesia & other CNS effects that are similar to those seen with morphine-like opioids.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 573
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
77
PharmaCompass offers a list of Dextropropoxyphene API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Dextropropoxyphene manufacturer or Dextropropoxyphene supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Dextropropoxyphene manufacturer or Dextropropoxyphene supplier.
PharmaCompass also assists you with knowing the Dextropropoxyphene API Price utilized in the formulation of products. Dextropropoxyphene API Price is not always fixed or binding as the Dextropropoxyphene Price is obtained through a variety of data sources. The Dextropropoxyphene Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Dextropropoxyphene manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Dextropropoxyphene, including repackagers and relabelers. The FDA regulates Dextropropoxyphene manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Dextropropoxyphene API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Dextropropoxyphene manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Dextropropoxyphene supplier is an individual or a company that provides Dextropropoxyphene active pharmaceutical ingredient (API) or Dextropropoxyphene finished formulations upon request. The Dextropropoxyphene suppliers may include Dextropropoxyphene API manufacturers, exporters, distributors and traders.
click here to find a list of Dextropropoxyphene suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Dextropropoxyphene DMF (Drug Master File) is a document detailing the whole manufacturing process of Dextropropoxyphene active pharmaceutical ingredient (API) in detail. Different forms of Dextropropoxyphene DMFs exist exist since differing nations have different regulations, such as Dextropropoxyphene USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Dextropropoxyphene DMF submitted to regulatory agencies in the US is known as a USDMF. Dextropropoxyphene USDMF includes data on Dextropropoxyphene's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Dextropropoxyphene USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Dextropropoxyphene suppliers with USDMF on PharmaCompass.
Dextropropoxyphene Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Dextropropoxyphene GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Dextropropoxyphene GMP manufacturer or Dextropropoxyphene GMP API supplier for your needs.
A Dextropropoxyphene CoA (Certificate of Analysis) is a formal document that attests to Dextropropoxyphene's compliance with Dextropropoxyphene specifications and serves as a tool for batch-level quality control.
Dextropropoxyphene CoA mostly includes findings from lab analyses of a specific batch. For each Dextropropoxyphene CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Dextropropoxyphene may be tested according to a variety of international standards, such as European Pharmacopoeia (Dextropropoxyphene EP), Dextropropoxyphene JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Dextropropoxyphene USP).
