
Synopsis
Synopsis
0
JDMF
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
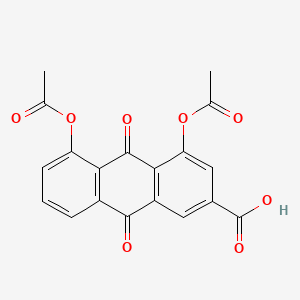

1. 1,8-diacetoxy-3-carboxyanthraquinone
2. 2-anthracenecarboxylic Acid, 4,5-bis(acetyloxy)-9,10-dihydro-9,10-dioxo-
3. 4,5-bis(acetyloxy)-9,10-dihydro-9,10-dioxo-2-anthracenecarboxylic Acid
4. 4,5-diacetoxyanthraquinone-2-carboxylic Acid
5. 9,10-dihydro-4,5-dihydroxy-9,10-dioxo-2-anthroic Acid, Diacetate
6. Ac-201
7. Ac-203
8. Artrodar
9. Diacerhein
10. Diacetyl-rhein
11. Diacetylrhein
12. Diora
13. Fisiodar
14. M-01ax21
15. M01ax21
16. Rhein Diacetate
17. Sf-277
18. Sf277
19. Verboril
20. Zondar
1. 13739-02-1
2. Diacerhein
3. Diacetylrhein
4. Artrodar
5. Fisiodar
6. 1,8-diacetoxy-3-carboxyanthraquinone
7. Diacerin
8. Sf-277
9. Verboril
10. Zondar
11. Diora
12. 4,5-diacetyloxy-9,10-dioxoanthracene-2-carboxylic Acid
13. M01ax21
14. Ac-201
15. 2-anthracenecarboxylic Acid, 4,5-bis(acetyloxy)-9,10-dihydro-9,10-dioxo-
16. 4,5-bis(acetyloxy)-9,10-dihydro-9,10-dioxo-2-anthracenecarboxylic Acid
17. 4,5-diacetoxyanthraquinone-2-carboxylic Acid
18. M-01ax21
19. Diacerhein; Diacetylrhein
20. Mfcd00468030
21. Nsc-758147
22. Mls000028577
23. 4hu6j11el5
24. 4,5-diacetoxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxylic Acid
25. 9,10-dihydro-4,5-dihydroxy-9,10-dioxo-2-anthroic Acid, Diacetate
26. 4,5-diacetylrhein
27. Ncgc00018274-04
28. Smr000058958
29. 4,5-bis(acetyloxy)-9,10-dioxo-9,10-dihydroanthracene-2-carboxylic Acid
30. Dsstox_cid_25636
31. Dsstox_rid_81017
32. Diacerein [inn]
33. Dsstox_gsid_45636
34. Diacereine [french]
35. Diacereinum [latin]
36. Diacereina [spanish]
37. 4,5-diacetoxy-9,10-diketo-anthracene-2-carboxylic Acid
38. 4,5-diacetyloxy-9,10-dioxo-2-anthracenecarboxylic Acid
39. Diacereina
40. Diacereine
41. Diacereinum
42. Rhein Diacetate
43. Rhein, Diacetate
44. Cas-13739-02-1
45. Sr-01000003156
46. Einecs 237-310-2
47. Brn 2184909
48. Unii-4hu6j11el5
49. Gesamtmatrix
50. Art 50
51. 1,8-diacetoxyanthraquinone-3-carboxylic Acid
52. Diacerein- Bio-x
53. Zondar (tn)
54. Spectrum_001876
55. Specplus_000643
56. 4,5-diacetoxy-9,10-dihydro-9,10-dioxo-2-anthrylcarbonsaeure
57. 9,10-dihydro-4,5-diacetoxy-9,10-2-anthracenecarboxylic Acid
58. Diacerein [mi]
59. Diacerein (usan/inn)
60. Diacerein [usan:inn]
61. Diacerein [usan]
62. 9,10-dihydro-4,5-dihydroxy-9,10-dioxo-2-anthroic Acid Diacetate
63. Spectrum2_000823
64. Spectrum3_000937
65. Spectrum4_001036
66. Spectrum5_001819
67. Diacerein Impurity Mixture
68. Diacerein [mart.]
69. Diacerein [who-dd]
70. Schembl25784
71. Kbiogr_001591
72. Kbioss_002400
73. Rhein Diacetate [mi]
74. 3-10-00-04790 (beilstein Handbook Reference)
75. Cid_26248
76. Chembl41286
77. Divk1c_006739
78. Spectrum1502010
79. Spbio_000745
80. 4,5-diacetoxy-9,10-dioxo-anthracene-2-carboxylic Acid
81. Diacerein [ep Monograph]
82. Diacerein, >=95% (hplc)
83. Dtxsid4045636
84. Bdbm32018
85. Chebi:94708
86. Gtpl10800
87. Kbio1_001683
88. Kbio2_002395
89. Kbio2_004963
90. Kbio2_007531
91. Kbio3_001974
92. Hms3652d06
93. Hms3714b20
94. Pharmakon1600-01502010
95. Bcp10834
96. Hy-n0283
97. Who 5371
98. Zinc3812842
99. Tox21_110856
100. Bbl011075
101. Ccg-40287
102. Nsc758147
103. S4267
104. Stk802271
105. 2-anthroic Acid, 9,10-dihydro-4,5-dihydroxy-9,10-dioxo-, Diacetate
106. Akos005622705
107. Tox21_110856_1
108. Db11994
109. Ks-5088
110. Kw-4800
111. Nsc 758147
112. Ncgc00018274-01
113. Ncgc00018274-02
114. Ncgc00018274-03
115. Ncgc00018274-05
116. Ncgc00022114-03
117. Am807992
118. Bd164367
119. Sbi-0052833.p002
120. B1726
121. D4061
122. Ft-0603096
123. Sw199355-2
124. D07270
125. Ab00053327_14
126. 739d021
127. A807252
128. Q413178
129. Sr-01000003156-2
130. Sr-01000003156-3
131. Sr-01000003156-4
132. W-108237
133. Diacerein, European Pharmacopoeia (ep) Reference Standard
134. 4,5-diacetoxy-9,10-dihydro-9,10-dioxoanthracene-2-carboxylic Acid
135. 4,5-diacetyloxy-9,10-bis(oxidanylidene)anthracene-2-carboxylic Acid
136. 4,5-bis(acetoxy)-9,10-dihydro-9,10-dioxo-2-anthracenecarboxylic Acid
137. 112118-18-0
| Molecular Weight | 368.3 g/mol |
|---|---|
| Molecular Formula | C19H12O8 |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 368.05321734 g/mol |
| Monoisotopic Mass | 368.05321734 g/mol |
| Topological Polar Surface Area | 124 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 683 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of osteoarthritis affecting the hip or knee.
Decreases inflammation and cartilage destruction and also corrects altered osteoblast acitivity.
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AX - Other antiinflammatory and antirheumatic agents, non-steroids
M01AX21 - Diacerein
Absorption
Bioavailability of 50-60%. Entirely converted to the active metabolite rhein [DB13174] before reaching systemic circulation.
Route of Elimination
37% excreted in urine and 53% in feces as estimated in rats.
Volume of Distribution
15-60L.
Clearance
Total CL is 1.5L/h and renal CL is 0.1L/h.
Entirely converted to rhien [DB13174] through double deacetylation before reaching systemic circulation. Rhein [DB13174] is further metabolized to rhein glucuronide and rhein sulfate.
4-10h.
Diacerein's active metabolite rhein [DB13174] reduces cartilage destruction by decreasing expression of matrix metalloproteinase (MMP)-1 and -3 as well as upregulating tissue inhibitor of matrix metalloproteinases which serve to reduce the activity of several MMPs. The anti-inflammatory action of rhein reduces the level of interleukin-1beta activity which plays a large role in reduction of extracellular matrix production, MMP activity, and continued inflammation. Rhein reduces abnormal osteoblast synthetic activity through an unknown mechanism.
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38766
Submission : 2023-09-15
Status : Active
Type : II

Certificate Number : CEP 2015-173 - Rev 03
Issue Date : 2024-12-03
Type : Chemical
Substance Number : 2409
Status : Valid

Date of Issue : 2022-08-11
Valid Till : 2025-06-26
Written Confirmation Number : WC-0054
Address of the Firm :

NDC Package Code : 59680-014
Start Marketing Date : 2024-04-09
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Huseeed Co., Ltd.
Registration Date : 2021-11-30
Registration Number : 20180515-209-J-27(6)
Manufacturer Name : Ami Lifesciences Pvt. Ltd.
Manufacturer Address : Block No. 82/B, ECP Road, At & Post. Karakhadi-391 450, Taluka:Padra, Dist.Vadodara, Gujarat, India
 Delivering Quality APIs, Drug Intermediates, and Specialty Chemicals to Over 50 Countries Across the Globe.
Delivering Quality APIs, Drug Intermediates, and Specialty Chemicals to Over 50 Countries Across the Globe.

Registrant Name : Hiple Co., Ltd.
Registration Date : 2019-07-24
Registration Number : 20180515-209-J-27(3)
Manufacturer Name : Ami Lifesciences Private Limited
Manufacturer Address : Block No. 82/B, ECP Road At & Post. Karakhadi, Tal-Padra, City : Karakhadi-391450, Dist : Vadodara, Gujarat State India

Registrant Name : Sungwoo Chemical Co., Ltd.
Registration Date : 2021-11-10
Registration Number : 20180515-209-J-27(5)
Manufacturer Name : Ami Lifesciences Pvt. Ltd.
Manufacturer Address : Block NO. 82/B, ECP Road, AT&Post : Karakhadi-391 450, Taluka:Padra, Dist. Vadodara, Gujarat, India

Registrant Name : Gapi Bio Co., Ltd.
Registration Date : 2018-08-27
Registration Number : 20180515-209-J-27(2)
Manufacturer Name : Ami Lifesciences Private Limited
Manufacturer Address : Block No.82/B, ECP Road, At & Post, Karakhadi, TAL-Pradra City : Karakhadi-391 450, Dist : Vadodara, Gujarat State India

Registrant Name : Hwail Pharmaceutical Co., Ltd.
Registration Date : 2018-08-14
Registration Number : 20180515-209-J-27(1)
Manufacturer Name : Ami Lifesciences Private Limited
Manufacturer Address : Block No.82/B, ECP Road, At & Post, Karakhadi, TAL-Pradra City : Karakhadi-391 450, Dist : Vadodara, Gujarat State India

Registrant Name : Aging Life Science Co., Ltd.
Registration Date : 2018-05-15
Registration Number : 20180515-209-J-27
Manufacturer Name : Ami Lifesciences Private Limited
Manufacturer Address : Block No.82/B, ECP Road, At & Post, Karakhadi, TAL-Pradra City : Karakhadi-391 450, Dist : Vadodara, Gujarat State India
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38766
Submission : 2023-09-15
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 23961
Submission : 2010-07-13
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 23960
Submission : 2010-07-13
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Certificate Number : CEP 2015-173 - Rev 03
Status : Valid
Issue Date : 2024-12-03
Type : Chemical
Substance Number : 2409
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2014-247 - Rev 00
Status : Valid
Issue Date : 2021-06-24
Type : Chemical
Substance Number : 2409

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R0-CEP 2014-248 - Rev 00
Status : Expired
Issue Date : 2016-07-21
Type : Chemical
Substance Number : 2409

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2017-148 - Rev 03
Status : Valid
Issue Date : 2024-03-26
Type : Chemical
Substance Number : 2409

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R0-CEP 2021-483 - Rev 00
Status : Valid
Issue Date : 2023-05-30
Type : Chemical
Substance Number : 2409

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Date of Issue : 2022-08-11
Valid Till : 2025-06-26
Written Confirmation Number : WC-0054
Address of the Firm : Block No. 82/B, ECP Road, At & Post. Karakhadi, Tal-Padra, City Karakhadi -39145...
Date of Issue : 2013-08-30
Valid Till : 2016-07-02
Written Confirmation Number : WC-249
Address of the Firm : A-36, MIDC Industrial Area, Patalganga, Village-Kaire. Tal-Khalapur, Dist. Raiga...

Date of Issue : 2019-08-05
Valid Till : 2022-07-21
Written Confirmation Number : WC-0075A
Address of the Firm : Plot No.138, GIDC, At & Post Ankleshwar, Dist- Bharuch, Gujarat, India

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Registrant Name : Sungwoo Chemical Co., Ltd.
Registration Date : 2021-11-10
Registration Number : 20180515-209-J-27(5)
Manufacturer Name : Ami Lifesciences Pvt. Ltd.
Manufacturer Address : Block NO. 82/B, ECP Road, AT&Post : Karakhadi-391 450, Taluka:Padra, Dist. Vadodara, ...
Registrant Name : Hiple Co., Ltd.
Registration Date : 2019-07-24
Registration Number : 20180515-209-J-27(3)
Manufacturer Name : Ami Lifesciences Private Lim...
Manufacturer Address : Block No. 82/B, ECP Road At & Post. Karakhadi, Tal-Padra, City : Karakhadi-391450, Di...
Registrant Name : Gapi Bio Co., Ltd.
Registration Date : 2018-08-27
Registration Number : 20180515-209-J-27(2)
Manufacturer Name : Ami Lifesciences Private Lim...
Manufacturer Address : Block No.82/B, ECP Road, At & Post, Karakhadi, TAL-Pradra City : Karakhadi-391 450, D...
Registrant Name : Hwail Pharmaceutical Co., Ltd.
Registration Date : 2018-08-14
Registration Number : 20180515-209-J-27(1)
Manufacturer Name : Ami Lifesciences Private Lim...
Manufacturer Address : Block No.82/B, ECP Road, At & Post, Karakhadi, TAL-Pradra City : Karakhadi-391 450, D...
Registrant Name : Aging Life Science Co., Ltd.
Registration Date : 2018-05-15
Registration Number : 20180515-209-J-27
Manufacturer Name : Ami Lifesciences Private Lim...
Manufacturer Address : Block No.82/B, ECP Road, At & Post, Karakhadi, TAL-Pradra City : Karakhadi-391 450, D...
Registrant Name : Huseeed Co., Ltd.
Registration Date : 2021-11-30
Registration Number : 20180515-209-J-27(6)
Manufacturer Name : Ami Lifesciences Pvt. Ltd.
Manufacturer Address : Block No. 82/B, ECP Road, At & Post. Karakhadi-391 450, Taluka:Padra, Dist.Vadodara, ...
Registrant Name : Myungmoon Pharmaceutical Co., Ltd.
Registration Date : 2019-09-24
Registration Number : 20180515-209-J-27(4)
Manufacturer Name : Ami Lifesciences Private Lim...
Manufacturer Address : Block No. 82/B, ECP Road At & Post. Karakhadi, Tal-Padra, City : Karakhadi-391450, Di...
Registrant Name : Dongbang FTL Co., Ltd.
Registration Date : 2018-10-17
Registration Number : 20181017-209-J-144
Manufacturer Name : [Product manufacturer ③] Z...
Manufacturer Address : [Raw Material Manufacturer ③] No.1, Badu Road, Tiantai Industrial Park, Tiantai, Ch...

Registrant Name : Hana Pharmaceutical Co., Ltd.
Registration Date : 2022-01-06
Registration Number : 20220106-209-J-1197
Manufacturer Name : Hana Pharmaceutical Co., Ltd...
Manufacturer Address : 133, Yakjakgongdan 3-gil, Hyangnam-eup, Hwaseong-si, Gyeonggi-do

Registrant Name : Samoh Pharmaceutical Co., Ltd.
Registration Date : 2021-11-23
Registration Number : 20211123-209-J-1075
Manufacturer Name : Interpretation of SA of CV
Manufacturer Address : Guillermo Marconi No. 16, Fraccionamiento Parque Industrial Cuamatla, CP 54730, Cuaut...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
About the Company : LGM Pharma is a global leader in sourcing hard-to-find APIs and intermediates for the pharmaceutical and biotech industries. LGM is also a full service CDMO providing formulation, ...
 Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
About the Company : Gonane Pharma, is a contract pharmaceutical company located in Gujarat, India, specializing in the manufacturing and marketing of Corticosteroids, Hormones, Antivirals, and Oncolog...
About the Company : Jai Radhe Sales was founded in 1999 as an out-of-the-box distribution firm specializing in the global supply of high-quality pharmaceutical ingredients. The firm provides complete ...
About the Company : Ami Lifesciences, established in 2006, is one of the fastest growing API manufacturing companies in India. Specializing in cardiovascular, anti-diabetic, CNS, and respiratory thera...
 Delivering Quality APIs, Drug Intermediates, and Specialty Chemicals to Over 50 Countries Across the Globe.
Delivering Quality APIs, Drug Intermediates, and Specialty Chemicals to Over 50 Countries Across the Globe.
About the Company : Zeal MediPharma is a globally recognized Star One Export House, serving customers in over 50 countries for more than two decades. We specialize in sourcing and exporting high-quali...
About the Company : Amuna Pharmaceuticals is an emerging contract research and manufacturing organization (CRMO) that caters to the needs of small, mid-size, and large pharmaceutical and biotechnology...

About the Company : We provide quality products and services, consistently, reliably responsibly and continuously applying some of the world’s most difficult to handle chemical technologies. Synth...

About the Company : Atomgrid is a global company that specializes in the manufacturing and sourcing of specialty chemicals. The company manages end-to-end manufacturing and sourcing needs, covering R&...

About the Company : Chorus Labs was incepted in 2009, with a clear vision to master API manufacturing. The company is foraying aggressively in to the pharma market in developing and commercializing pr...

About the Company : Rumit group of companies is a young, dynamic & Robustly growing company established in 1993. We specialise in pharmaceutical raw material, Bulk API, dye stuff, dyes intermediates &...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
AC-201 is a novel small molecule inhibitor of TYK2/JAK1 with high activity, selectivity, and safety window. The preclinical studies have shown that AC-201 effectively binds to the pseudo kinase domain (JH2) of TYK2/JAK1.
Lead Product(s): Diacerein
Therapeutic Area: Dermatology Brand Name: AC-201
Study Phase: IND EnablingProduct Type: Microorganism
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 24, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Diacerein
Therapeutic Area : Dermatology
Highest Development Status : IND Enabling
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : AC-201 is a novel small molecule inhibitor of TYK2/JAK1 with high activity, selectivity, and safety window. The preclinical studies have shown that AC-201 effectively binds to the pseudo kinase domain (JH2) of TYK2/JAK1.
Product Name : AC-201
Product Type : Microorganism
Upfront Cash : Inapplicable
May 24, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Strategic partnership grants Winhealth Pharma exclusive right to develop and commercialize rare disease asset AC-203 in mainland China, Hong Kong and Macao for indications including hereditary epidermolysis bullosa (EB), bullous pemphigoid and other skin diseases.
Lead Product(s): Diacerein
Therapeutic Area: Rare Diseases and Disorders Brand Name: AC-203
Study Phase: Phase IIProduct Type: Other Small Molecule
Sponsor: Hong Kong Winhealth Pharma Group
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Partnership June 01, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Diacerein
Therapeutic Area : Rare Diseases and Disorders
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Hong Kong Winhealth Pharma Group
Deal Size : Undisclosed
Deal Type : Partnership
Winhealth Pharma and TWiB Enter into Strategic Licensing Partnership on AC-203
Details : Strategic partnership grants Winhealth Pharma exclusive right to develop and commercialize rare disease asset AC-203 in mainland China, Hong Kong and Macao for indications including hereditary epidermolysis bullosa (EB), bullous pemphigoid and other skin...
Product Name : AC-203
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
June 01, 2021

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Octavius has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Octavius has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Regulatory Info :
Registration Country : India
Brand Name : Diacerein
Dosage Form : Capsule
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Nepal
Brand Name : DCR 50
Dosage Form : Capsule
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Nepal

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name :
Dosage Form : TABLET
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Egypt
Brand Name : Pentacerin
Dosage Form : Capsule
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Egypt

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name : Arthocerin – A
Dosage Form : Tablet
Dosage Strength : 50MG; 100MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Diacerein; Glucosamine Hydrochloride
Brand Name : Arthocerin – DG
Dosage Form : Tablet
Dosage Strength : 50MG; 1500MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Capsule
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name : OSTIKAR
Dosage Form : CAPSULE
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : India

Regulatory Info :
Registration Country : Spain
Brand Name : Glizolan 50Mg 30 Capsules
Dosage Form : Capsule
Dosage Strength : 50 Mg/Capsule
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Spain

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Spain
Brand Name : Galaxdar 50Mg 30 Capsules
Dosage Form : Capsule
Dosage Strength : 50 Mg/Capsule
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Spain

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Regulatory Info :
Registration Country : Spain
Brand Name : Glizolan 50Mg 30 Capsules
Dosage Form : Capsule
Dosage Strength : 50 Mg/Capsule
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Spain

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Spain
Brand Name : Galaxdar 50Mg 30 Capsules
Dosage Form : Capsule
Dosage Strength : 50 Mg/Capsule
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Spain

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Spain
Brand Name : Diacereina Normon 50Mg 30 Capsules
Dosage Form : Capsule
Dosage Strength : 50 Mg/Capsule
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Spain

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Octavius has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Octavius has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Regulatory Info :
Registration Country : India
Brand Name : Diacerein
Dosage Form : Capsule
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Octavius has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Octavius has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Packaging :
Regulatory Info :
Dosage : Capsule
Dosage Strength : 50MG
Brand Name : Diacerein
Approval Date :
Application Number :
Registration Country : India
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Nepal
Brand Name : DCR 50
Dosage Form : Capsule
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Nepal

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Capsule
Dosage Strength : 50MG
Brand Name : DCR 50
Approval Date :
Application Number :
Registration Country : Nepal

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name :
Dosage Form : TABLET
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info : Generic
Dosage : TABLET
Dosage Strength : 50MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Diacerein; Dimethyl Sulfone; Glucosamine
Brand Name :
Dosage Form : Tablet
Dosage Strength : 50MG; 250MG; 75MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Diacerein; Dimethyl Sulfone; Glucosamine
Dosage : Tablet
Dosage Strength : 50MG; 250MG; 75MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Capsule
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Capsule
Dosage Strength : 50MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Egypt
Brand Name : Pentacerin
Dosage Form : Capsule
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Egypt

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Capsule
Dosage Strength : 50MG
Brand Name : Pentacerin
Approval Date :
Application Number :
Registration Country : Egypt

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name : Arthocerin – A
Dosage Form : Tablet
Dosage Strength : 50MG; 100MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 50MG; 100MG
Brand Name : Arthocerin – A
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Diacerein; Glucosamine Hydrochloride
Brand Name : Arthocerin – DG
Dosage Form : Tablet
Dosage Strength : 50MG; 1500MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Diacerein; Glucosamine Hydrochloride
Dosage : Tablet
Dosage Strength : 50MG; 1500MG
Brand Name : Arthocerin – DG
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Capsule
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Capsule
Dosage Strength : 50MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name : OSTIKAR
Dosage Form : CAPSULE
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info : Generic
Dosage : CAPSULE
Dosage Strength : 50MG
Brand Name : OSTIKAR
Approval Date :
Application Number :
Registration Country : India

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

Reply
12 Apr 2025
Reply
28 Dec 2023
Reply
16 Oct 2023
Reply
15 Jul 2023
Reply
01 Jun 2023
Reply
22 Apr 2023
Reply
18 Nov 2022
Reply
30 Sep 2022
Reply
25 Jul 2022
Reply
07 Jun 2022
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Reply
15 Jun 2023
Reply
11 Jul 2022
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
43
PharmaCompass offers a list of Diacerein API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Diacerein manufacturer or Diacerein supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Diacerein manufacturer or Diacerein supplier.
PharmaCompass also assists you with knowing the Diacerein API Price utilized in the formulation of products. Diacerein API Price is not always fixed or binding as the Diacerein Price is obtained through a variety of data sources. The Diacerein Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A DIACERIN manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of DIACERIN, including repackagers and relabelers. The FDA regulates DIACERIN manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. DIACERIN API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of DIACERIN manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A DIACERIN supplier is an individual or a company that provides DIACERIN active pharmaceutical ingredient (API) or DIACERIN finished formulations upon request. The DIACERIN suppliers may include DIACERIN API manufacturers, exporters, distributors and traders.
click here to find a list of DIACERIN suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A DIACERIN DMF (Drug Master File) is a document detailing the whole manufacturing process of DIACERIN active pharmaceutical ingredient (API) in detail. Different forms of DIACERIN DMFs exist exist since differing nations have different regulations, such as DIACERIN USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A DIACERIN DMF submitted to regulatory agencies in the US is known as a USDMF. DIACERIN USDMF includes data on DIACERIN's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The DIACERIN USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of DIACERIN suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a DIACERIN Drug Master File in Korea (DIACERIN KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of DIACERIN. The MFDS reviews the DIACERIN KDMF as part of the drug registration process and uses the information provided in the DIACERIN KDMF to evaluate the safety and efficacy of the drug.
After submitting a DIACERIN KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their DIACERIN API can apply through the Korea Drug Master File (KDMF).
click here to find a list of DIACERIN suppliers with KDMF on PharmaCompass.
A DIACERIN CEP of the European Pharmacopoeia monograph is often referred to as a DIACERIN Certificate of Suitability (COS). The purpose of a DIACERIN CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of DIACERIN EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of DIACERIN to their clients by showing that a DIACERIN CEP has been issued for it. The manufacturer submits a DIACERIN CEP (COS) as part of the market authorization procedure, and it takes on the role of a DIACERIN CEP holder for the record. Additionally, the data presented in the DIACERIN CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the DIACERIN DMF.
A DIACERIN CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. DIACERIN CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of DIACERIN suppliers with CEP (COS) on PharmaCompass.
A DIACERIN written confirmation (DIACERIN WC) is an official document issued by a regulatory agency to a DIACERIN manufacturer, verifying that the manufacturing facility of a DIACERIN active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting DIACERIN APIs or DIACERIN finished pharmaceutical products to another nation, regulatory agencies frequently require a DIACERIN WC (written confirmation) as part of the regulatory process.
click here to find a list of DIACERIN suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing DIACERIN as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for DIACERIN API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture DIACERIN as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain DIACERIN and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a DIACERIN NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of DIACERIN suppliers with NDC on PharmaCompass.
DIACERIN Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of DIACERIN GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right DIACERIN GMP manufacturer or DIACERIN GMP API supplier for your needs.
A DIACERIN CoA (Certificate of Analysis) is a formal document that attests to DIACERIN's compliance with DIACERIN specifications and serves as a tool for batch-level quality control.
DIACERIN CoA mostly includes findings from lab analyses of a specific batch. For each DIACERIN CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
DIACERIN may be tested according to a variety of international standards, such as European Pharmacopoeia (DIACERIN EP), DIACERIN JP (Japanese Pharmacopeia) and the US Pharmacopoeia (DIACERIN USP).
