
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Australia

0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
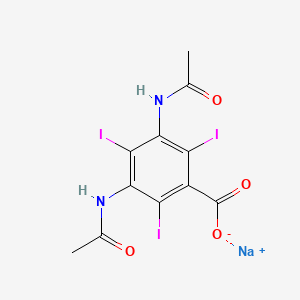

1. Sodium Diatrizoate
2. 737-31-5
3. Sodium Amidotrizoate
4. Triombrine
5. Hypaque Sodium
6. Diatrizoic Acid Sodium Salt
7. Sodium 3,5-diacetamido-2,4,6-triiodobenzoate
8. Triombrin
9. Natrii Amidotrizoas
10. Vascoray
11. Conray 35
12. Hypaque Cysto
13. Hpaque - Cysto
14. Amidotrizoato Sodico
15. Histopaque
16. Hypaque Sodium 20%
17. Urovist Sodium
18. Diatrizoate Sodium Salt
19. Amidotrizoate De Sodium
20. Benzoic Acid, 3,5-bis(acetylamino)-2,4,6-triiodo-, Monosodium Salt
21. Hypaque 50
22. Md 50
23. Sodium Diacetyldiaminetriiodobenzoate
24. Sodium Amidotrizoate (inn)
25. Urographing 370
26. Monosodium 3,5-diacetamido-2,4,6-triiodobenzoate
27. Urografic Acid, Sodium Salt
28. Diatrizoate Sodium [usp]
29. Sodium Amidotrizoate [inn]
30. Ml 216
31. Win 8308-3
32. 3,5-diacetamido-2,4,6-triiodobenzoic Acid Sodium Salt
33. Md-50
34. Mls000069526
35. Chebi:53692
36. V5403h8vg7
37. Gastrografin
38. 3,5-diacetylamino-2,4,6-trijodbenzosaeure Natrium
39. Nsc-61815
40. Smr000058586
41. Diatrizoate Sodium (usp)
42. Sodium;3,5-diacetamido-2,4,6-triiodobenzoate
43. Diatrizoic Acid Sodium Salt;sodium Amidotrizoate
44. Benzoic Acid, 3,5-diacetamido-2,4,6-triiodo-, Monosodium Salt
45. Urovist Sodium 300
46. Radioselectan
47. Urovist 300
48. Natrii Amidotrizoas [inn-latin]
49. Amidotrizoato Sodico [inn-spanish]
50. Einecs 212-004-1
51. Nsc 61815
52. Amidotrizoate De Sodium [inn-french]
53. Renografin 76 Injectable (veterinary)
54. Unii-v5403h8vg7
55. Hsdb 8078
56. 3,5-diacetamido-2,4,6-triiodobenzoic Acid, Sodium Salt
57. Urovist Sodium (tn)
58. 3,5-diacetylamino-2,4,6-trijodbenzosaeure Natrium [german]
59. Benzoic Acid, 3,5-diacetamido-2,4,6-triiodo-, Sodium Salt
60. Opera_id_214
61. Dsstox_cid_2912
62. Sodium Diatrizoate [ban]
63. Dsstox_rid_76785
64. Dsstox_gsid_22912
65. Schembl37116
66. Chembl1200581
67. Dtxsid7022912
68. Hy-b0926a
69. Diatrizoate Sodium [vandf]
70. Diatrizoate Sodium [who-ip]
71. Tox21_301439
72. Diatrizoic Acid Sodium Salt Dihydrate
73. Sodium Amidotrizoate [mart.]
74. Sodium Amidotrizoate [who-dd]
75. Sodium Amidotrizoate [who-ip]
76. Akos024371067
77. Ccg-270302
78. Diatrizoate Sodium [green Book]
79. Diatrizoate Sodium [orange Book]
80. Diatrizoic Acid Sodium Salt [mi]
81. Ncgc00255327-01
82. Cas-737-31-5
83. Diatrizoate Sodium [usp Monograph]
84. Sodium Amidotrizoate [ep Impurity]
85. Natrii Amidotrizoas [who-ip Latin]
86. Renocal Component Diatrizoate Sodium
87. Sodium Amidotrizoate [ep Monograph]
88. Renovist Component Diatrizoate Sodium
89. Angiovist Component Diatrizoate Sodium
90. Cs-0013563
91. Ft-0603095
92. Diatrizoate Sodium Component Of Renocal
93. Gastrovist Component Diatrizoate Sodium
94. Renografin Component Diatrizoate Sodium
95. Sodium3,5-diacetamido-2,4,6-triiodobenzoate
96. A17075
97. D01013
98. D97593
99. Diatrizoate Sodium Component Of Renovist
100. Gastrografin Component Diatrizoate Sodium
101. Diatrizoate Sodium Component Of Angiovist
102. Diatrizoate Sodium Component Of Gastrovist
103. Diatrizoate Sodium Component Of Renografin
104. Md-gastroview Component Diatrizoate Sodium
105. Diatrizoate Sodium Component Of Gastrografin
106. Diatrizoate Sodium Component Of Md-gastroview
107. Sodium 3,5-bis(acetylamino)-2,4,6-triiodobenzoate
108. 3,5-diacetamido-2,4,6-tiiodobenzoic Acid Sodium Salt
109. Q27104811
| Molecular Weight | 635.89 g/mol |
|---|---|
| Molecular Formula | C11H8I3N2NaO4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 635.7516 g/mol |
| Monoisotopic Mass | 635.7516 g/mol |
| Topological Polar Surface Area | 98.3 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 396 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
GLOFIL-125 (Sodium Iothalamate I-125 Injection) is indicated for evaluation of glomerular filtration in the diagnosis or monitoring of patients with renal disease.
US Natl Inst Health; DailyMed. Current Medication Information for Glofil-125 (iothalamate sodium, I-125) Injection (June 2007). Available from, as of July 23, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2c07ce91-cc83-45e3-bd2b-f923a9c8286c
Contrast Media
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
As in the use of any radioactive material, care should be taken to minimize radiation exposure to the patient, consistent with proper patient management, and to insure minimum radiation exposure to occupational workers.
US Natl Inst Health; DailyMed. Current Medication Information for Glofil-125 (iothalamate sodium, I-125) Injection (June 2007). Available from, as of July 23, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2c07ce91-cc83-45e3-bd2b-f923a9c8286c
Rapid or bolus-like injections should be avoided.
US Natl Inst Health; DailyMed. Current Medication Information for Glofil-125 (iothalamate sodium, I-125) Injection (June 2007). Available from, as of July 23, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2c07ce91-cc83-45e3-bd2b-f923a9c8286c
Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides.
US Natl Inst Health; DailyMed. Current Medication Information for Glofil-125 (iothalamate sodium, I-125) Injection (June 2007). Available from, as of July 23, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2c07ce91-cc83-45e3-bd2b-f923a9c8286c
For more Drug Warnings (Complete) data for Iothalamate (8 total), please visit the HSDB record page.
Radioiodine is excreted in human milk during lactation. It is not known whether GLOFIL-125 is excreted in human milk.
US Natl Inst Health; DailyMed. Current Medication Information for Glofil-125 (iothalamate sodium, I-125) Injection (June 2007). Available from, as of July 23, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2c07ce91-cc83-45e3-bd2b-f923a9c8286c
The renal clearance of sodium iothalamate in man closely approximates that of inulin. The compound is cleared by glomerular filtration without tubular secretion or reabsorption.
US Natl Inst Health; DailyMed. Current Medication Information for Glofil-125 (iothalamate sodium, I-125) Injection (June 2007). Available from, as of July 23, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2c07ce91-cc83-45e3-bd2b-f923a9c8286c
Following infusion administration of I-125 iothalamate, the effective half-life is about 0.07 days.
US Natl Inst Health; DailyMed. Current Medication Information for Glofil-125 (iothalamate sodium, I-125) Injection (June 2007). Available from, as of July 23, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2c07ce91-cc83-45e3-bd2b-f923a9c8286c

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Global Sales Information

Market Place

ABOUT THIS PAGE
24
PharmaCompass offers a list of Diatrizoate Sodium API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Diatrizoate Sodium manufacturer or Diatrizoate Sodium supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Diatrizoate Sodium manufacturer or Diatrizoate Sodium supplier.
PharmaCompass also assists you with knowing the Diatrizoate Sodium API Price utilized in the formulation of products. Diatrizoate Sodium API Price is not always fixed or binding as the Diatrizoate Sodium Price is obtained through a variety of data sources. The Diatrizoate Sodium Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Diatrizoate Sodium manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Diatrizoate Sodium, including repackagers and relabelers. The FDA regulates Diatrizoate Sodium manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Diatrizoate Sodium API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Diatrizoate Sodium manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Diatrizoate Sodium supplier is an individual or a company that provides Diatrizoate Sodium active pharmaceutical ingredient (API) or Diatrizoate Sodium finished formulations upon request. The Diatrizoate Sodium suppliers may include Diatrizoate Sodium API manufacturers, exporters, distributors and traders.
click here to find a list of Diatrizoate Sodium suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Diatrizoate Sodium DMF (Drug Master File) is a document detailing the whole manufacturing process of Diatrizoate Sodium active pharmaceutical ingredient (API) in detail. Different forms of Diatrizoate Sodium DMFs exist exist since differing nations have different regulations, such as Diatrizoate Sodium USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Diatrizoate Sodium DMF submitted to regulatory agencies in the US is known as a USDMF. Diatrizoate Sodium USDMF includes data on Diatrizoate Sodium's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Diatrizoate Sodium USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Diatrizoate Sodium suppliers with USDMF on PharmaCompass.
A Diatrizoate Sodium written confirmation (Diatrizoate Sodium WC) is an official document issued by a regulatory agency to a Diatrizoate Sodium manufacturer, verifying that the manufacturing facility of a Diatrizoate Sodium active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Diatrizoate Sodium APIs or Diatrizoate Sodium finished pharmaceutical products to another nation, regulatory agencies frequently require a Diatrizoate Sodium WC (written confirmation) as part of the regulatory process.
click here to find a list of Diatrizoate Sodium suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Diatrizoate Sodium as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Diatrizoate Sodium API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Diatrizoate Sodium as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Diatrizoate Sodium and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Diatrizoate Sodium NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Diatrizoate Sodium suppliers with NDC on PharmaCompass.
Diatrizoate Sodium Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Diatrizoate Sodium GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Diatrizoate Sodium GMP manufacturer or Diatrizoate Sodium GMP API supplier for your needs.
A Diatrizoate Sodium CoA (Certificate of Analysis) is a formal document that attests to Diatrizoate Sodium's compliance with Diatrizoate Sodium specifications and serves as a tool for batch-level quality control.
Diatrizoate Sodium CoA mostly includes findings from lab analyses of a specific batch. For each Diatrizoate Sodium CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Diatrizoate Sodium may be tested according to a variety of international standards, such as European Pharmacopoeia (Diatrizoate Sodium EP), Diatrizoate Sodium JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Diatrizoate Sodium USP).
