
Synopsis
Synopsis
0
KDMF
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
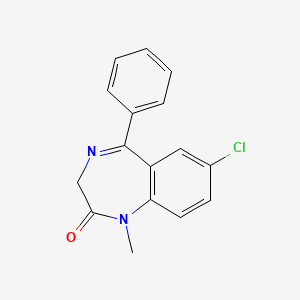

1. 7-chloro-1,3-dihydro-1-methyl-5-phenyl-2h-1,4-benzodiazepin-2-one
2. Apaurin
3. Diazemuls
4. Faustan
5. Relanium
6. Seduxen
7. Sibazon
8. Stesolid
9. Valium
1. Valium
2. 439-14-5
3. Ansiolisina
4. Diazemuls
5. Apaurin
6. Faustan
7. Relanium
8. Seduxen
9. Sibazon
10. Stesolid
11. Methyldiazepinone
12. Ansiolin
13. Apozepam
14. Atensine
15. Bensedin
16. Bialzepam
17. Calmocitene
18. Calmpose
19. Ceregulart
20. Condition
21. Diacepan
22. Diazepan
23. Diazetard
24. Dipezona
25. Domalium
26. Kiatrium
27. Liberetas
28. Neurolytril
29. Paranten
30. Quetinil
31. Quiatril
32. Quievita
33. Relaminal
34. Renborin
35. Ruhsitus
36. Seduksen
37. Serenack
38. Serenamin
39. Serenzin
40. Stesolin
41. Tensopam
42. Tranimul
43. Tranqdyn
44. Tranquirit
45. Unisedil
46. Valitran
47. Valrelease
48. Alboral
49. Aliseum
50. Amiprol
51. Armonil
52. Assival
53. Atilen
54. Cercine
55. Dialag
56. Diapam
57. Diastat
58. Dienpax
59. Duksen
60. Eridan
61. Eurosan
62. Freudal
63. Frustan
64. Gihitan
65. Lembrol
66. Levium
67. Morosan
68. Paxate
69. Plidan
70. Saromet
71. Sedipam
72. Setonil
73. Sonacon
74. Umbrium
75. Vatran
76. Velium
77. Duxen
78. Lamra
79. Paxel
80. Solis
81. Valeo
82. Vival
83. Vivol
84. Zipan
85. Noan
86. Usempax Ap
87. Methyl Diazepinone
88. Q-pam
89. Centrazepam
90. Kabivitrum
91. La-iii
92. Evacalm
93. Gewacalm
94. Horizon
95. Novazam
96. Tranquase
97. Valaxona
98. Valiquid
99. Dialar
100. Dipam
101. Dizac
102. Paceum
103. La Iii
104. An-ding
105. E-pam
106. Jinpanfan
107. Servizepam
108. Simasedan
109. Antenex
110. Arzepam
111. Diapax
112. Drenian
113. Ducene
114. Kratium
115. Nellium
116. Nerozen
117. Zepaxid
118. Doval
119. Paxum
120. Sipam
121. Vazen
122. Placidox 5
123. Ro 5-2807
124. Wy-3467
125. 7-chloro-1-methyl-5-phenyl-3h-1,4-benzodiazepin-2-one
126. 7-chloro-1,3-dihydro-1-methyl-5-phenyl-2h-1,4-benzodiazepin-2-one
127. D-pam
128. Nsc-77518
129. 7-chloro-1-methyl-5-phenyl-1,3-dihydro-2h-1,4-benzodiazepin-2-one
130. Diacepin
131. Diazepam Civ
132. 2h-1,4-benzodiazepin-2-one, 7-chloro-1,3-dihydro-1-methyl-5-phenyl-
133. Faustan,
134. 7-chloro-1-methyl-5-phenyl-3h-1,4-benzodiazepin-2(1h)-one
135. 7-chloro-1-methyl-2-oxo-5-phenyl-3h-1,4-benzodiazepine
136. 7-chloro-1-methyl-5-phenyl-2h-1,4-benzodiazepin-2-one
137. Chembl12
138. Q3jtx2q7tu
139. Nsc-169897
140. 1-methyl-5-phenyl-7-chloro-1,3-dihydro-2h-1,4-benzodiazepin-2-one
141. Nrl-1
142. Ro-5-2807
143. Dzp
144. Methyldiazepinone, Pharmaceutical
145. Chebi:49575
146. Apo-diazepam
147. Wy 3467
148. Diazepam Intensol
149. La 111
150. Ro-52807
151. 7-chloro-1-methyl-5-3h-1,4-benzodiazepin-2(1h)-one
152. Ncgc00178168-02
153. Calmociteno
154. Alupram
155. Ansilive
156. Anxicalm
157. Anxionil
158. Benzopin
159. Betapam
160. Britazepam
161. Calmaven
162. Chuansuan
163. Desconet
164. Desloneg
165. Diaceplex
166. Diapine
167. Diaquel
168. Diatran
169. Diazepin
170. Disopam
171. Faustal
172. Gradual
173. Iazepam
174. Medipam
175. Mentalium
176. Metamidol
177. Nervium
178. Nivalen
179. Nixtensyn
180. Notense
181. Novodipam
182. Ortopsique
183. Paralium
184. Prozepam
185. Psychopax
186. Radizepam
187. Reliver
188. Sedapam
189. Trankinon
190. Trazepam
191. Valuzepam
192. Vanconin
193. Baogin
194. Calmod
195. Caudel
196. Diazem
197. Lovium
198. Mandro
199. Parzam
200. Dipaz
201. Dupin
202. Gubex
203. Lizan
204. Pomin
205. Winii
206. Diazepam Nordic
207. Diazepam-lipuro
208. Diazepan Leo
209. Diazepam Elmu
210. Diazepam Fabra
211. Diazepam Stada
212. Dsstox_cid_406
213. Metil Gobanal
214. Diazepam Dak
215. Tranquo-tablinen
216. Diazepam Desitin
217. Euphorin P
218. Mandro-zep
219. Sico Relax
220. Diazepam Rectubes
221. S.a. R.l.
222. Elcion Cr
223. Placidox 2
224. Kratium 2
225. Diazepam-ratiopharm
226. Dsstox_rid_75566
227. Dsstox_gsid_20406
228. Placidox 10
229. Diazepamu [polish]
230. Diazepam-eurogenerics
231. Diazepamum
232. Diazepamu
233. Mandrozep
234. Plumiaz
235. Tensium
236. Tranquo-puren
237. Diastat Acudial
238. Diazepamum [inn-latin]
239. 7-chloro-1-methyl-5-phenyl-1,3-dihydro-benzo[e][1,4]diazepin-2-one
240. 7-chloro-1-methyl-5-phenyl-2,3-dihydro-1h-1,4-benzodiazepin-2-one
241. Wln: T67 Gnv Jn Ihj Cg G1 Kr
242. Cas-439-14-5
243. Smr000058398
244. Ccris 6009
245. Best [pharaceutical]
246. Hsdb 3057
247. Methyldiazepinone (pharmaceutical)
248. 2h-1, 7-chloro-1,3-dihydro-1-methyl-5-phenyl-
249. Einecs 207-122-5
250. Unii-q3jtx2q7tu
251. La-111
252. 1-methyl-5-phenyl-7-chloro-1,4-benzodiazepin-2-one
253. 7-chloro-1-methyl-5-phenyl-1,4-benzodiazepin-2-one
254. Nsc 169897
255. Brn 0754371
256. Anlin
257. Dea No. 2765
258. Pro Pam
259. Diastat (tn)
260. Valium (tn)
261. Valtoco
262. Diazepam [usan:usp:inn:ban:jan]
263. Diazepam [hsdb]
264. Diazepam [iarc]
265. Diazepam [usan]
266. Diazepam [inn]
267. Diazepam [jan]
268. Diazepam [mi]
269. Diazepam [vandf]
270. Spectrum3_001780
271. Spectrum4_000576
272. Spectrum5_001890
273. Diazepam [mart.]
274. Diazepam [who-dd]
275. Bidd:pxr0158
276. Oprea1_126223
277. Schembl21442
278. Bspbio_003279
279. Kbiogr_001012
280. 5-24-04-00300 (beilstein Handbook Reference)
281. Mls000759402
282. Mls001424086
283. Bidd:gt0105
284. Diazepam [green Book]
285. Divk1c_000967
286. Diazepam (jp17/usp/inn)
287. Diazepam [orange Book]
288. Diazepam Civ [usp-rs]
289. Mono (2-ethylhexyl) Phthalate
290. Gtpl3364
291. S.a.r.l.
292. Zinc6427
293. Diazepam [ep Monograph]
294. Diazepam [usp Monograph]
295. Dtxsid4020406
296. Hms503a15
297. Kbio1_000967
298. Kbio3_002780
299. Diazepam 0.1 Mg/ml In Methanol
300. Diazepam 1.0 Mg/ml In Methanol
301. Ninds_000967
302. Hms2051n04
303. Hms3393n04
304. Bcp23002
305. Nsc77518
306. Tox21_113071
307. Tox21_202458
308. 7-chloro-1-methyl-5-phenyl-1h-benzo[e][1,4]diazepin-2(3h)-one
309. Bdbm50000766
310. Nsc169897
311. Stk735517
312. Akos003367969
313. Diazepam 1000 Microg/ml In Methanol
314. Tox21_113071_1
315. Ab02310
316. Bcp9000199
317. Ccg-100997
318. Cs-0653
319. Db00829
320. Nc00247
321. Ro 5-2805
322. Idi1_000967
323. Ncgc00178168-01
324. Ncgc00178168-03
325. Ncgc00178168-04
326. Ncgc00260007-01
327. Ac-10561
328. Hy-17027
329. Bcp0726000176
330. Db-051179
331. C06948
332. D00293
333. A826456
334. Q210402
335. Valium; Ansiolisina; Diazemuls; Apaurin; Faustan
336. Brd-k16508793-001-01-8
337. Diazepam, European Pharmacopoeia (ep) Reference Standard
338. 7-chloranyl-1-methyl-5-phenyl-3h-1,4-benzodiazepin-2-one
339. 7-chloro-1-methyl-5-phenyl-3h-1,4-benzodiazepin-2-one.
340. 7-chloro-1-methyl-5-phenyl-1h-1,4-benzodiazepine-2(3h)on
341. Diazepam, United States Pharmacopeia (usp) Reference Standard
342. 7-chloro-1-methyl-5-phenyl-1,3-dihydro-[1,4]benzodiazepin-2-one
343. 7 -chloro-1,3-dihydro-1-methyl-5-phenyl-2h-1,4-benzodiazepin-2-one
344. 7-chloro-1,3-dihydro-1-methyl-5-phenyl-2h -1,4-benzodiazepin-2-one
345. 7-chloro-1,3-dihydro-1-methyl-5-phenyl-2h-1,4-benzodiazepine-2-one
346. 7-chloro-1-methyl-1,3-dihydro-5-phenyl-2h-1,4-benzodiazepin-2-one
347. 7-chloro-1-methyl-5-phenyl-2-oxo-2,3-dihydro-1h-benzo[f]-1,4-diazepine
348. Diazepam For System Suitability, European Pharmacopoeia (ep) Reference Standard
349. Diazepam Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
350. Diazepam Solution, 1.0 Mg/ml In Methanol, Analytical Standard, For Drug Analysis
351. 11100-37-1
| Molecular Weight | 284.74 g/mol |
|---|---|
| Molecular Formula | C16H13ClN2O |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 284.0716407 g/mol |
| Monoisotopic Mass | 284.0716407 g/mol |
| Topological Polar Surface Area | 32.7 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 403 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 10 | |
|---|---|
| Drug Name | Diastat |
| PubMed Health | Diazepam (Rectal) |
| Drug Classes | Anticonvulsant |
| Drug Label | Diazepam rectal gel rectal delivery system is a non-sterile diazepam gel provided in a prefilled, unit-dose, rectal delivery system. Diazepam rectal gel contains 5 mg/mL diazepam, propylene glycol, ethyl alcohol (10%), hydroxypropyl methylcellulose,... |
| Active Ingredient | Diazepam |
| Dosage Form | Gel |
| Route | Rectal |
| Strength | 2.5mg/0.5ml (5mg/ml) |
| Market Status | Prescription |
| Company | Valeant |
| 2 of 10 | |
|---|---|
| Drug Name | Diastat acudial |
| PubMed Health | Diazepam |
| Drug Classes | Antianxiety, Anticonvulsant, Skeletal Muscle Relaxant |
| Drug Label | Diazepam is a benzodiazepine derivative. The chemical name ofdiazepam is 7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one. It is a colorless to light yellow crystalline compound, insoluble in water. The empirical formula is C16H13C... |
| Active Ingredient | Diazepam |
| Dosage Form | Gel |
| Route | Rectal |
| Strength | 20mg/4ml (5mg/ml); 10mg/2ml (5mg/ml) |
| Market Status | Prescription |
| Company | Valeant |
| 3 of 10 | |
|---|---|
| Drug Name | Diazepam |
| PubMed Health | Diazepam (By mouth) |
| Drug Classes | Antianxiety, Anticonvulsant, Skeletal Muscle Relaxant |
| Drug Label | Each 5 mL of Oral Solution contains:Diazepam........................................................... 5 mgEach mL of IntensolTM Oral Solution (Concentrate) contains:Diazepam............................................................ 5 mgAlcohol...... |
| Active Ingredient | Diazepam |
| Dosage Form | Injectable; Tablet; Concentrate; Solution |
| Route | Injection; Oral |
| Strength | 5mg; 5mg/ml; 2mg; 10mg; 5mg/5ml |
| Market Status | Prescription |
| Company | Lannett Holdings; Watson Labs; Vintage Pharms; Ivax Sub Teva Pharms; Hospira; Mylan; Roxane; Barr |
| 4 of 10 | |
|---|---|
| Drug Name | Diazepam intensol |
| PubMed Health | Diazepam |
| Drug Classes | Antianxiety, Anticonvulsant, Skeletal Muscle Relaxant |
| Drug Label | Valium (diazepam) is a benzodiazepine derivative. The chemical name of diazepam is 7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one. It is a colorless to light yellow crystalline compound, insoluble in water. The empirical formula is... |
| Active Ingredient | Diazepam |
| Dosage Form | Concentrate |
| Route | Oral |
| Strength | 5mg/ml |
| Market Status | Prescription |
| Company | Roxane |
| 5 of 10 | |
|---|---|
| Drug Name | Valium |
| Active Ingredient | Diazepam |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 2mg; 10mg |
| Market Status | Prescription |
| Company | Roche |
| 6 of 10 | |
|---|---|
| Drug Name | Diastat |
| PubMed Health | Diazepam (Rectal) |
| Drug Classes | Anticonvulsant |
| Drug Label | Diazepam rectal gel rectal delivery system is a non-sterile diazepam gel provided in a prefilled, unit-dose, rectal delivery system. Diazepam rectal gel contains 5 mg/mL diazepam, propylene glycol, ethyl alcohol (10%), hydroxypropyl methylcellulose,... |
| Active Ingredient | Diazepam |
| Dosage Form | Gel |
| Route | Rectal |
| Strength | 2.5mg/0.5ml (5mg/ml) |
| Market Status | Prescription |
| Company | Valeant |
| 7 of 10 | |
|---|---|
| Drug Name | Diastat acudial |
| PubMed Health | Diazepam |
| Drug Classes | Antianxiety, Anticonvulsant, Skeletal Muscle Relaxant |
| Drug Label | Diazepam is a benzodiazepine derivative. The chemical name ofdiazepam is 7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one. It is a colorless to light yellow crystalline compound, insoluble in water. The empirical formula is C16H13C... |
| Active Ingredient | Diazepam |
| Dosage Form | Gel |
| Route | Rectal |
| Strength | 20mg/4ml (5mg/ml); 10mg/2ml (5mg/ml) |
| Market Status | Prescription |
| Company | Valeant |
| 8 of 10 | |
|---|---|
| Drug Name | Diazepam |
| PubMed Health | Diazepam (By mouth) |
| Drug Classes | Antianxiety, Anticonvulsant, Skeletal Muscle Relaxant |
| Drug Label | Each 5 mL of Oral Solution contains:Diazepam........................................................... 5 mgEach mL of IntensolTM Oral Solution (Concentrate) contains:Diazepam............................................................ 5 mgAlcohol...... |
| Active Ingredient | Diazepam |
| Dosage Form | Injectable; Tablet; Concentrate; Solution |
| Route | Injection; Oral |
| Strength | 5mg; 5mg/ml; 2mg; 10mg; 5mg/5ml |
| Market Status | Prescription |
| Company | Lannett Holdings; Watson Labs; Vintage Pharms; Ivax Sub Teva Pharms; Hospira; Mylan; Roxane; Barr |
| 9 of 10 | |
|---|---|
| Drug Name | Diazepam intensol |
| PubMed Health | Diazepam |
| Drug Classes | Antianxiety, Anticonvulsant, Skeletal Muscle Relaxant |
| Drug Label | Valium (diazepam) is a benzodiazepine derivative. The chemical name of diazepam is 7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one. It is a colorless to light yellow crystalline compound, insoluble in water. The empirical formula is... |
| Active Ingredient | Diazepam |
| Dosage Form | Concentrate |
| Route | Oral |
| Strength | 5mg/ml |
| Market Status | Prescription |
| Company | Roxane |
| 10 of 10 | |
|---|---|
| Drug Name | Valium |
| Active Ingredient | Diazepam |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 2mg; 10mg |
| Market Status | Prescription |
| Company | Roche |
Adjuvants, Anesthesia; Anesthetics, Intravenous; Anti-Anxiety Agents, Benzodiazepine; Anticonvulsants; Antiemetics; GABA Modulators; Muscle Relaxants, Central; Sedatives, Nonbarbiturate
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Diazepam shares the actions of other benzodiazepines. The drug is used preoperatively to relieve anxiety and provide sedation, light anesthesia, and anterograde amnesia; as an adjunct during endoscopy to relieve anxiety and provide sedation, light anesthesia, and anterograde amnesia; for the management of agitation associated with acute alcohol withdrawal; as an adjunct for the relief of acute, painful musculoskeletal conditions; to manage skeletal muscle spasticity such as reflex spasm secondary to local pathology (e.g., trauma, inflammation), spasticity caused by upper motor neuron disorders (e.g., cerebral palsy, paraplegia), athetosis, stiff-man syndrome, or tetanus; and for the management of anxiety disorders or for the short-term relief of symptoms of anxiety. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic. The efficacy of diazepam for long-term use (i.e., longer than 4 months) as an anxiolytic has not been evaluated. /Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2576
Diazepam has been used orally to prevent night terrors. Although not recommended by the manufacturer, parenteral diazepam is used to reduce the requirements for opiate analgesics and produce anterograde amnesia during labor and delivery. The drug has been used parenterally to manage neonatal opiate withdrawal. /NOT included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2576
Diazepam is also used IV or rectally as an anticonvulsant, and IV diazepam or lorazepam generally are considered the drugs of choice for termination of status epilepticus. /Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2576
For more Therapeutic Uses (Complete) data for DIAZEPAM (10 total), please visit the HSDB record page.
Since Valium has a central nervous system depressant effect, patients should be advised against the simultaneous ingestion of alcohol and other CNS-depressant drugs during Valium therapy
US Natl Inst Health; DailyMed. Current Medication Information for VALIUM (diazepam) tablet (January 2008). Available from, as of November 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=18533
Diazepam is subject to Schedule IV control under the Controlled Substances Act of 1970. Abuse and dependence of benzodiazepines have been reported. Addiction-prone individuals (such as drug addicts or alcoholics) should be under careful surveillance when receiving diazepam or other psychotropic agents because of the predisposition of such patients to habituation and dependence. Once physical dependence to benzodiazepines have developed, termination of treatment will be accompanied by withdrawal symptoms. The risk is more pronounced in patients on long-term therapy.
US Natl Inst Health; DailyMed. Current Medication Information for VALIUM (diazepam) tablet (January 2008). Available from, as of November 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=18533
A transient syndrome whereby the symptoms that led to treatment with Valium recur in an enhanced form. This may occur upon discontinuation of treatment. It may be accompanied by other reactions including mood changes, anxiety, and restlessness. Since the risk of withdrawal phenomena and rebound phenomena is greater after abrupt discontinuation of treatment, it is recommended that the dosage be decreased gradually.
US Natl Inst Health; DailyMed. Current Medication Information for VALIUM (diazepam) tablet (January 2008). Available from, as of November 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=18533
Withdrawal symptoms, similar in character to those noted with barbiturates and alcohol have occurred following abrupt discontinuance of diazepam. These withdrawal symptoms may consist of tremor, abdominal and muscle cramps, vomiting, sweating, headache, muscle pain, extreme anxiety, tension, restlessness, confusion and irritability. In severe cases, the following symptoms may occur: derealization, depersonalization, hyperacusis, numbness and tingling of the extremities, hypersensitivity to light, noise and physical contact, hallucinations or epileptic seizures. The more severe withdrawal symptoms have usually been limited to those patients who had received excessive doses over an extended period of time. Generally milder withdrawal symptoms (e.g., dysphoria and insomnia) have been reported following abrupt discontinuance of benzodiazepines taken continuously at therapeutic levels for several months. Consequently, after extended therapy, abrupt discontinuation should generally be avoided and a gradual dosage tapering schedule followed. Chronic use (even at therapeutic doses) may lead to the development of physical dependence: discontinuation of the therapy may result in withdrawal or rebound phenomena.
US Natl Inst Health; DailyMed. Current Medication Information for VALIUM (diazepam) tablet (January 2008). Available from, as of November 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=18533
For more Drug Warnings (Complete) data for DIAZEPAM (24 total), please visit the HSDB record page.
In general, diazepam is useful in the symptomatic management of mild to moderate degrees of anxiety in conditions dominated by tension, excitation, agitation, fear, or aggressiveness such as may occur in psychoneurosis, anxiety reactions due to stress conditions, and anxiety states with somatic expression. Moreover, in acute alcoholic withdrawal, diazepam may be useful in the symptomatic relief of acute agitation, tremor, and impending acute delirium tremens. Furthermore, diazepam is a useful adjunct for the relief of skeletal muscle spasm due to reflex spasm to local pathologies, such as inflammation of the muscle and joints or secondary to trauma; spasticity caused by upper motor neuron disorders, such as cerebral palsy and paraplegia; athetosis and the rare "stiff man syndrome". Particular label information from the United Kingdom also lists particular age-specific indications, including for adults: (1) The short-term relief (2-4 weeks) only, of anxiety which is severe, disabling, or subjecting the individual to unacceptable distress, occurring alone or in association with insomnia or short-term psychosomatic, organic or psychotic illness, (2) cerebral palsy, (3) muscle spasm, (4) as an adjunct to certain types of epilepsy (eg. myoclonus), (5) symptomatic treatment of acute alcohol withdrawal, (6) as oral premedication for the nervous dental patient, and (7) for premedication before surgery. In the same UK label information, diazepam is indicated in children for: (1) control of tension and irritability in cerebral spasticity in selected cases, (2) as an adjunct to the control of muscle spasm in tetanus, and for (3) oral premedication. A diazepam nasal spray is indicated in patients 6 years and older to treat intermittent, stereotypic episodes of frequent seizure activity that are different than the patient's usual seizure pattern.
FDA Label
Diazepam is a benzodiazepine that exerts anxiolytic, sedative, muscle- relaxant, anticonvulsant and amnestic effects. Most of these effects are thought to result from facilitation of the action of gamma aminobutyric acid (GABA), an inhibitory neurotransmitter in the central nervous system.
Anti-Anxiety Agents
Agents that alleviate ANXIETY, tension, and ANXIETY DISORDERS, promote sedation, and have a calming effect without affecting clarity of consciousness or neurologic conditions. ADRENERGIC BETA-ANTAGONISTS are commonly used in the symptomatic treatment of anxiety but are not included here. (See all compounds classified as Anti-Anxiety Agents.)
Adjuvants, Anesthesia
Agents that are administered in association with anesthetics to increase effectiveness, improve delivery, or decrease required dosage. (See all compounds classified as Adjuvants, Anesthesia.)
Anesthetics, Intravenous
Ultrashort-acting anesthetics that are used for induction. Loss of consciousness is rapid and induction is pleasant, but there is no muscle relaxation and reflexes frequently are not reduced adequately. Repeated administration results in accumulation and prolongs the recovery time. Since these agents have little if any analgesic activity, they are seldom used alone except in brief minor procedures. (From AMA Drug Evaluations Annual, 1994, p174) (See all compounds classified as Anesthetics, Intravenous.)
Antiemetics
Drugs used to prevent NAUSEA or VOMITING. (See all compounds classified as Antiemetics.)
Hypnotics and Sedatives
Drugs used to induce drowsiness or sleep or to reduce psychological excitement or anxiety. (See all compounds classified as Hypnotics and Sedatives.)
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
GABA Modulators
Substances that do not act as agonists or antagonists but do affect the GAMMA-AMINOBUTYRIC ACID receptor-ionophore complex. GABA-A receptors (RECEPTORS, GABA-A) appear to have at least three allosteric sites at which modulators act: a site at which BENZODIAZEPINES act by increasing the opening frequency of GAMMA-AMINOBUTYRIC ACID-activated chloride channels; a site at which BARBITURATES act to prolong the duration of channel opening; and a site at which some steroids may act. GENERAL ANESTHETICS probably act at least partly by potentiating GABAergic responses, but they are not included here. (See all compounds classified as GABA Modulators.)
Muscle Relaxants, Central
A heterogeneous group of drugs used to produce muscle relaxation, excepting the neuromuscular blocking agents. They have their primary clinical and therapeutic uses in the treatment of muscle spasm and immobility associated with strains, sprains, and injuries of the back and, to a lesser degree, injuries to the neck. They have been used also for the treatment of a variety of clinical conditions that have in common only the presence of skeletal muscle hyperactivity, for example, the muscle spasms that can occur in MULTIPLE SCLEROSIS. (From Smith and Reynard, Textbook of Pharmacology, 1991, p358) (See all compounds classified as Muscle Relaxants, Central.)
N05BA01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N05BA01
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
N - Nervous system
N05 - Psycholeptics
N05B - Anxiolytics
N05BA - Benzodiazepine derivatives
N05BA01 - Diazepam
Absorption
After oral administration, it is considered that diazepam is rapidly and completely absorbed from the gastrointestinal tract as >90% of diazepam is absorbed and the average time to achieve peak plasma concentrations is 1 1.5 hours with a range of 0.25 to 2.5 hours. Absorption is delayed and decreased when administered with a moderate fat meal. In the presence of food mean lag times are approximately 45 minutes as compared with 15 minutes when fasting. There is also an increase in the average time to achieve peak concentrations to about 2.5 hours in the presence of food as compared with 1.25 hours when fasting. This results in an average decrease in Cmax of 20% in addition to a 27% decrease in AUC (range 15% to 50%) when administered with food.
Route of Elimination
Diazepam and its metabolites are excreted mainly in the urine, predominantly as their glucuronide conjugates.
Volume of Distribution
In young healthy males, the volume of distribution at steady-state is 0.8 to 1.0 L/kg.
Clearance
The clearance of diazepam is 20 to 30 mL/min in young adults.
Diazepam rectal gel is well absorbed following rectal administration, reaching peak plasma concentrations in 1.5 hours. The absolute bioavailability of Diazepam rectal gel relative to Valium injectable is 90%. The volume of distribution of Diazepam rectal gel is calculated to be approximately 1 L/kg. ... Both diazepam and its major active metabolite desmethyldiazepam bind extensively to plasma proteins (95-98%).
US Natl Inst Health; DailyMed. Current Medication Information for diastat (diazepam) gel (October 2007). Available from, as of November 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=5533
The concentration of diazepam in plasma and saliva and its binding to plasma protein was determined in normal subjects receiving 10 mg single oral dose over an 8 hr period. A linear relationship was found between diazepam concentration in plasma and that in both mixed and parotid saliva, over plasma concentrations ranging from 196-74.8 ug/mL. Results indicate that there is no significant difference between parotid saliva and mixed saliva concentrations over a period of 8 hr after a single oral dose of diazepam and that appearance of diazepam in saliva may provide an alternate, noninvasive method of determining plasma diazepam levels.
PMID:710030 DIGREGORIO GJ ET AL; CLIN PHARMACOL THER 24 (DEC): 720-5 (1978)
The rate of transplacental passage of diazepam was studied in 33 cases of cephalic presentation where operative forceps delivery was indicated by intrauterine hypoxia or by prolonged second stage of labor. Thirty mg of diazepam was injected iv immediately before delivery either during uterine contractions or in the relaxation period. It was concluded that transfer of diazepam from mother to fetus appears to be delayed when iv injection is given during uterine contractions.
PMID:699483 HARAM K ET AL; CLIN PHARMACOL THER 24 (NOV): 590-9 (1978)
Diazepam and its metabolites are highly bound to plasma proteins (diazepam 98%). Diazepam and its metabolites cross the blood-brain and placental barriers and are also found in breast milk in concentrations approximately one tenth of those in maternal plasma (days 3 to 9 post-partum).
US Natl Inst Health; DailyMed. Current Medication Information for VALIUM (diazepam) tablet (January 2008). Available from, as of November 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=18533
For more Absorption, Distribution and Excretion (Complete) data for DIAZEPAM (11 total), please visit the HSDB record page.
Diazepam is N-demethylated by CYP3A4 and 2C19 to the active metabolite N-desmethyldiazepam, and is hydroxylated by CYP3A4 to the active metabolite temazepam. N-desmethyldiazepam and temazepam are both further metabolized to oxazepam. Temazepam and oxazepam are further largely eliminated by way of conjugation to glucuronic acid via glucuronidation. Furthermore, oxidation of diazepam is mediated by cytochrome P450 isozymes; formation of desmethyl-diazepam mainly by CYP2C19 and CYP3A and 3-hydroxy-diazepam (temazepam) and oxazepam by CYP3A. Because CYP2C19 is polymorphic, extensive metabolizers (EMs), and poor metabolizers (PMs) of diazepam can be distinguished. PMs of diazepam showed significantly lower clearance (12 vs 26 mL/min) and longer elimination half-life (88 vs 41 h) of diazepam than EMs after a single oral dose. Also, PMs had lower clearance, higher AUC and longer elimination half-life of desmethyl-diazepam.
Diazepam is N-demethylated by CYP3A4 and 2C19 to the active metabolite N-desmethyldiazepam, and is hydroxylated by CYP3A4 to the active metabolite temazepam. N-desmethyldiazepam and temazepam are both further metabolized to oxazepam. Temazepam and oxazepam are largely eliminated by glucuronidation.
US Natl Inst Health; DailyMed. Current Medication Information for VALIUM (diazepam) tablet (January 2008). Available from, as of November 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=18533
/Investigators/ observed variations in the metabolism of diazepam in Wistar rats. /The authors/ studied these variations carefully, and found that the variations are dimorphic and about 17% of male Wistar rats examined showed two times higher diazepam metabolic activities in their liver microsomes than the rest of animals at the substrate concentrations less than 5 uM. /They were/ classified as extensive metabolizer and poor metabolizer of diazepam. No sex difference was observed in the frequency of appearance of extensive metabolizer. Activities of the primary metabolic pathways of diazepam were examined to elucidate the cause of this polymorphism in male Wistar rats. No significant differences were observed in activities of neither diazepam 3-hydroxylation or N-desmethylation between extensive metabolizer and poor metabolizer rats, while activity of diazepam p-hydroxylation was markedly (more than 200 times) higher in extensive metabolizer rats, indicating that this reaction is responsible for the polymorphism of diazepam metabolism in Wistar rats. We examined the expression levels of CYP2D1, which was reported to catalyze diazepam p-hydroxylation in Wistar rats to find no differences in the expression levels of CYP2D1 between extensive metabolizer and PM rats. The kinetic study on diazepam metabolism in male Wistar rats revealed that extensive metabolizer rats had markedly higher V(max) and smaller K(m) in diazepam p-hydroxylation than those of poor metabolizer rats, indicating the presence of high affinity high capacity p-hydroxylase enzyme in extensive metabolizer rats. As a consequence, at low concentrations of diazepam, major pathways of diazepam metabolism were p-hydroxylation and 3-hydroxylation in male extensive metabolizer rats, while in male poor metabolizer rats, 3-hydroxylation followed by N-desmethylation. Due to this kinetic nature of p-hydroxylase activity, extensive metabolizer rats had markedly higher total CL(int) of diazepam than that of poor metabolizer rats. Polymorphism in diazepam metabolism in humans is well documented, but this is the first report revealing the presence of the polymorphism in diazepam metabolism in rats. The current results infer polymorphic expression of new diazepam p-hydroxylating enzyme with lower K(m) than CYP2D1 in extensive metabolizer Wistar rats.
PMID:15067703 Saito K et al; J Pharm Sci 93 (5): 1271-8 (2004)
Diazepam has known human metabolites that include Temazepam and nordiazepam.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Diazepam has a biphasic half-life with an initial rapid distribution phase followed by a prolonged terminal elimination phase of 1 or 2 days; its action is further prolonged by the even longer half-life of 2-5 days of its principal active metabolite, desmethyldiazepam (nordiazepam), the relative proportion of which increases in the body on long-term administration. The plasma half-life of diazepam is prolonged in neonates, in the elderly, and in patients with kidney or liver disease.
... The distributive (alpha) half-life of diazepam is about 1 hr, while the elimination (beta) half-life is about 1.5 days initially and even longer after prolonged treatment.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 423
In full term infants, elimination half-lives around 30 hours have been reported, with a longer average half-life of 54 hours reported in premature infants of 28 - 34 weeks gestational age and 8 - 81 days post-partum. In both premature and full term infants the active metabolite desmethyldiazepam shows evidence of continued accumulation compared to children. Longer half-lives in infants may be due to incomplete maturation of metabolic pathways
US Natl Inst Health; DailyMed. Current Medication Information for VALIUM (diazepam) tablet (January 2008). Available from, as of November 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=18533
Elimination half-life increases by approximately 1 hour for each year of age beginning with a half-life of 20 hours at 20 years of age.
US Natl Inst Health; DailyMed. Current Medication Information for VALIUM (diazepam) tablet (January 2008). Available from, as of November 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=18533
In mild and moderate cirrhosis, average half-life is increased. The average increase has been variously reported from 2-fold to 5-fold, with individual half-lives over 500 hours reported. ... Mean half-life is also prolonged with hepatic fibrosis to 90 hours (range 66 - 104 hours), with chronic active hepatitis to 60 hours (range 26 - 76 hours), and with acute viral hepatitis to 74 hours (range 49 - 129).
US Natl Inst Health; DailyMed. Current Medication Information for VALIUM (diazepam) tablet (January 2008). Available from, as of November 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=18533
For more Biological Half-Life (Complete) data for DIAZEPAM (7 total), please visit the HSDB record page.
Diazepam is a benzodiazepine tranquilliser with anticonvulsant, sedative, muscle relaxant and amnesic properties. Benzodiazepines, such as diazepam, bind to receptors in various regions of the brain and spinal cord. This binding increases the inhibitory effects of gamma-aminobutyric acid (GABA). GABAs functions include CNS involvement in sleep induction. Also involved in the control of hypnosis, memory, anxiety, epilepsy and neuronal excitability.
Diazepam is a benzodiazepine that exerts anxiolytic, sedative, muscle-relaxant, anticonvulsant and amnestic effects. Most of these effects are thought to result from a facilitation of the action of gamma aminobutyric acid (GABA), an inhibitory neurotransmitter in the central nervous system.
US Natl Inst Health; DailyMed. Current Medication Information for VALIUM (diazepam) tablet (January 2008). Available from, as of November 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=18533
Benzodiazepines are widely used in clinical anesthesia as premedication, but also to induce general anesthesia. Recent in vitro studies suggest that gamma-aminobutyric acid type A receptors, harboring a classical high-affinity benzodiazepine binding site, possess another "nonclassical" binding site for benzodiazepines. At present, it is unclear if, and to what extent, this novel nonclassical binding site is of relevance for the actions of benzodiazepines in the central nervous system. Because neocortex is involved in mediating the sedative and hypnotic properties of general anesthetics, ... the actions of diazepam /were quantified/ over a wide range of concentrations (from 10 nM up to 100 uM) in organotypic slice cultures using extracellular multiunit recordings of spontaneous action potential activity. Up to a concentration of 6.25 uM, diazepam reduced the activity of neocortical neurons, approaching a maximum of approximately 20%. This action was nullified by the benzodiazepine antagonist flumazenil. At concentrations >12.5 uM, diazepam evoked a second concentration-dependent dampening of network activity. Unlike the low concentration effect, this high concentration component was resistant to flumazenil. Diazepam induced a biphasic attenuation of spontaneous action potential firing of neocortical neurons. Low to moderate concentrations caused a monotonic, mild depression that is mediated via the classical binding site as it is antagonized by flumazenil. However, the effects of diazepam observed at high concentrations were not affected by flumazenil. Hence, these findings support the concept of at least 2 different binding sites for benzodiazepines on gamma-aminobutyric acid type A receptors. Furthermore, /these/ results are consistent with the hypothesis that the classical high-affinity binding site mediates low-dose diazepam actions, such as amnesia, anxiolysis, and sedation, while a second, nonclassical and independent site contributes to the anesthetic effects of diazepam, such as hypnosis and immobility.
PMID:20889946 Drexler B et al; Anesth Analg 111 (6): 1394-9 (2010)
Benzodiazepine site agonists or inverse agonists enhance or reduce gamma-aminobutyric acid(A) (GABA(A)) receptor-mediated inhibition of neurons, respectively. Recently, it was demonstrated that the point mutation gamma 2F77I causes a drastic change in the affinity of a variety of benzodiazepine agonists or inverse agonists in receptor binding studies. Here we investigated the potency and efficacy of 10 benzodiazepine site ligands from 6 structural classes in wild-type and gamma 2F77I point mutated recombinant GABA(A) receptors composed of alpha 1 beta 3 gamma 2, alpha 2 beta 3 gamma 2, alpha 3 beta 3 gamma 2, alpha 4 beta 3 gamma 2, alpha 5 beta 3 gamma 2, and alpha 6 beta 3 gamma 2 subunits. Results indicate that the effects of the benzodiazepine site ligands zolpidem, zopiclone, Cl218872, L-655,708 and DMCM were nearly completely eliminated in all mutated receptors up to a 1 microM concentration. The effects of bretazenil, Ro15-1788 or abecarnil were eliminated in some, but not all mutated receptors, suggesting that the gamma 2F77I mutation differentially influences the actions of these ligands in different receptor subtypes. In addition, this point mutation also influences the efficacy of diazepam for enhancing GABA-induced chloride flux, suggesting that the amino acid residue gamma 2F77 might also be involved in the transduction of the effect of benzodiazepines from binding to gating.
PMID:20303942 Ramerstorfer J et al; Eur J Pharmacol 636 (1-3): 18-27 (2010)
Although the precise mechanism by which diazepam exerts its antiseizure effects is unknown, animal and in vitro studies suggest that diazepam acts to suppress seizures through an interaction with gamma-aminobutyric acid (GABA) receptors of the A-type (GABAA). GABA, the major inhibitory neurotransmitter in the central nervous system, acts at this receptor to open the membrane channel allowing chloride ions to flow into neurons. Entry of chloride ions causes an inhibitory potential that reduces the ability of neurons to depolarize to the threshold potential necessary to produce action potentials. Excessive depolarization of neurons is implicated in the generation and spread of seizures. It is believed that diazepam enhances the actions of GABA by causing GABA to bind more tightly to the GABAA receptor
US Natl Inst Health; DailyMed. Current Medication Information for diastat (diazepam) gel (October 2007). Available from, as of November 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=5533
For more Mechanism of Action (Complete) data for DIAZEPAM (8 total), please visit the HSDB record page.
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.

GDUFA
DMF Review : Reviewed
Rev. Date : 2012-10-24
Pay. Date : 2013-05-13
DMF Number : 3164
Submission : 1978-02-28
Status : Active
Type : II

Certificate Number : R1-CEP 2003-272 - Rev 03
Issue Date : 2023-04-17
Type : Chemical
Substance Number : 22
Status : Valid

Registration Number : 217MF10542
Registrant's Address : Viale Milano 26 36075 Montecchio Maggiore Vicenza, Italy
Initial Date of Registration : 2005-09-09
Latest Date of Registration :

NDC Package Code : 48087-0112
Start Marketing Date : 2014-05-26
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2018-07-13
Pay. Date : 2018-06-20
DMF Number : 1478
Submission : 1970-02-03
Status : Active
Type : II

Registration Number : 218MF10146
Registrant's Address : Via Curiel 34, 20067 Paulo, Milano, ITALY
Initial Date of Registration : 2006-01-31
Latest Date of Registration :

NDC Package Code : 12828-0013
Start Marketing Date : 1986-04-18
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2023-11-14
Pay. Date : 2023-09-26
DMF Number : 38844
Submission : 2023-09-27
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2021-07-08
Pay. Date : 2021-07-06
DMF Number : 21860
Submission : 2008-07-24
Status : Active
Type : II

Certificate Number : CEP 2008-195 - Rev 05
Issue Date : 2023-10-19
Type : Chemical
Substance Number : 22
Status : Valid

Date of Issue : 2022-06-08
Valid Till : 2025-06-25
Written Confirmation Number : WC-0107
Address of the Firm :

NDC Package Code : 64330-010
Start Marketing Date : 2008-07-24
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2021-11-24
Pay. Date : 2021-09-20
DMF Number : 33858
Submission : 2019-06-07
Status : Active
Type : II

Certificate Number : R0-CEP 2019-264 - Rev 02
Issue Date : 2023-08-16
Type : Chemical
Substance Number : 22
Status : Valid
Date of Issue : 2021-08-06
Valid Till : 2022-07-28
Written Confirmation Number : WC-0445A4
Address of the Firm :

NDC Package Code : 71554-118
Start Marketing Date : 2020-08-11
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT FOR HUMAN PRESCRIPTION COMPOUNDING

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 7025
Submission : 1987-06-18
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 6071
Submission : 1985-10-07
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
85
PharmaCompass offers a list of Diazepam API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Diazepam manufacturer or Diazepam supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Diazepam manufacturer or Diazepam supplier.
PharmaCompass also assists you with knowing the Diazepam API Price utilized in the formulation of products. Diazepam API Price is not always fixed or binding as the Diazepam Price is obtained through a variety of data sources. The Diazepam Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Diazepam manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Diazepam, including repackagers and relabelers. The FDA regulates Diazepam manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Diazepam API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Diazepam manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Diazepam supplier is an individual or a company that provides Diazepam active pharmaceutical ingredient (API) or Diazepam finished formulations upon request. The Diazepam suppliers may include Diazepam API manufacturers, exporters, distributors and traders.
click here to find a list of Diazepam suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Diazepam DMF (Drug Master File) is a document detailing the whole manufacturing process of Diazepam active pharmaceutical ingredient (API) in detail. Different forms of Diazepam DMFs exist exist since differing nations have different regulations, such as Diazepam USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Diazepam DMF submitted to regulatory agencies in the US is known as a USDMF. Diazepam USDMF includes data on Diazepam's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Diazepam USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Diazepam suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Diazepam Drug Master File in Japan (Diazepam JDMF) empowers Diazepam API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Diazepam JDMF during the approval evaluation for pharmaceutical products. At the time of Diazepam JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Diazepam suppliers with JDMF on PharmaCompass.
A Diazepam CEP of the European Pharmacopoeia monograph is often referred to as a Diazepam Certificate of Suitability (COS). The purpose of a Diazepam CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Diazepam EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Diazepam to their clients by showing that a Diazepam CEP has been issued for it. The manufacturer submits a Diazepam CEP (COS) as part of the market authorization procedure, and it takes on the role of a Diazepam CEP holder for the record. Additionally, the data presented in the Diazepam CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Diazepam DMF.
A Diazepam CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Diazepam CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Diazepam suppliers with CEP (COS) on PharmaCompass.
A Diazepam written confirmation (Diazepam WC) is an official document issued by a regulatory agency to a Diazepam manufacturer, verifying that the manufacturing facility of a Diazepam active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Diazepam APIs or Diazepam finished pharmaceutical products to another nation, regulatory agencies frequently require a Diazepam WC (written confirmation) as part of the regulatory process.
click here to find a list of Diazepam suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Diazepam as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Diazepam API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Diazepam as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Diazepam and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Diazepam NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Diazepam suppliers with NDC on PharmaCompass.
Diazepam Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Diazepam GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Diazepam GMP manufacturer or Diazepam GMP API supplier for your needs.
A Diazepam CoA (Certificate of Analysis) is a formal document that attests to Diazepam's compliance with Diazepam specifications and serves as a tool for batch-level quality control.
Diazepam CoA mostly includes findings from lab analyses of a specific batch. For each Diazepam CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Diazepam may be tested according to a variety of international standards, such as European Pharmacopoeia (Diazepam EP), Diazepam JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Diazepam USP).
