
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
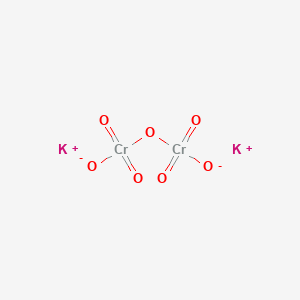

1. Bichromate, Potassium
2. Dichromate, Potassium
3. K2cr2o7
4. Potassium Bichromate
1. 7778-50-9
2. Potassium Bichromate
3. Kaliumdichromat
4. Potassium Dichromate(vi)
5. Dipotassium Bichromate
6. Dipotassium Dichromate
7. Dichromic Acid Dipotassium Salt
8. Dipotassium Dichromium Heptaoxide
9. Chromium Potassium Oxide
10. Iopezite
11. Chebi:53444
12. T4423s18fm
13. Mfcd00011367
14. Dipotassium;oxido-(oxido(dioxo)chromio)oxy-dioxochromium
15. Dsstox_cid_5948
16. Dsstox_rid_77973
17. Dsstox_gsid_25948
18. Caswell No. 690
19. Kaliumdichromat [german]
20. Chromium, Reference Standard Solution
21. Kalium Bichromicum
22. Srm 935a
23. Cas-7778-50-9
24. Ccris 2409
25. Hsdb 1238
26. Einecs 231-906-6
27. Nsc 77372
28. K2cr2o7
29. Epa Pesticide Chemical Code 068302
30. Unii-t4423s18fm
31. Kali Bichromicum
32. Potassium Dichromate(2-)
33. Epitope Id:119680
34. Ec 231-906-6
35. Chromic Acid (h2cr2o7) Potassium Salt (1:2)
36. Potassium Dichromate Solution
37. Potassium Dichromate Acs Grade
38. Potassium Dichromate [chromium And Chromium Compounds]
39. Kali Bichromicum [hpus]
40. Dtxsid5025948
41. Potassium Dichromate [hsdb]
42. Potassium Dichromate [vandf]
43. Tox21_111009
44. Tox21_202307
45. Potassium Dichromate [mart.]
46. Potassium Dichromate, Puriss., 99%
47. Potassium Dichromate [who-dd]
48. Akos024418771
49. Potassium Dichromate(vi) [mi]
50. Potassium Dichromate, Ar, >=99.9%
51. Potassium Dichromate, Lr, >=99.5%
52. Potassium Dichromate Solution, 1/60 M
53. Ncgc00090755-01
54. Ncgc00259856-01
55. Potassium Dichromate, Bioxtra, >=99.5%
56. Ft-0698964
57. Potassium Dichromate, Acs Reagent, >=99.0%
58. Potassium Dichromate, 0.1n Standardized Solution
59. Potassium Dichromate, Bioultra, >=99.5% (rt)
60. Potassium Dichromate, Reagentplus(r), >=99.5%
61. Chromic Acid (h2cr2o7), Potassium Salt (1:2)
62. Potassium Dichromate, 0.25n Standardized Solution
63. Potassium Dichromate, 99.99% Trace Metals Basis
64. Potassium Dichromate, Saj First Grade, >=99.5%
65. Q239729
66. Potassium Dichromate, 0.025n Standardized Solution
67. Potassium Dichromate, Jis Special Grade, >=99.5%
68. Potassium Dichromate, Yanagishima Pharmaceutical Co. Ltd.
69. Potassium Dichromate, Nist(r) Srm(r) 136f, Oxidimetric Standard
70. Potassium Dichromate, Nist(r) Srm(r) 935a, Uv Absorbance Standard
71. Potassium Dichromate, Puriss. P.a., Acs Reagent, Reag. Iso, Reag. Ph. Eur., >=99.8%
72. Chromium Standard Solution, Suitable For Atomic Absorption Spectrometry, 1 Mg/ml Cr, 1000 Ppm Cr
73. Potassium Dichromate, Certified Reference Material For Titrimetry, Certified By Bam According To Iso 17025, >=99.5%
| Molecular Weight | 294.18 g/mol |
|---|---|
| Molecular Formula | Cr2K2O7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 0 |
| Exact Mass | 293.772825 g/mol |
| Monoisotopic Mass | 293.772825 g/mol |
| Topological Polar Surface Area | 124 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 194 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Anti-Infective Agents, Local; Astringents; Caustics; Dyes
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Potassium dichromate is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of March 17, 2016: https://clinicaltrials.gov/ct2/results?term=potassium+dichromate&Search=Search
Medication (vet): caustic
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Cambridge, UK: Royal Society of Chemistry, 2013., p. 1419
MEDICATION (VET): HAS BEEN USED IN ANTITUSSIVE & LYMPHANGITIS MIXTURES; 20 PPM IN FISH TANK WATER TO CONTROL PROTOZOA & FLUKES
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 473
4 OR 5. 4= VERY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 50-500 MG/KG; BETWEEN 1 TEASPOON & 1 OZ FOR 70 KG PERSON (150 LB); 5= EXTREMELY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 5-50 MG/KG; BETWEEN 7 DROPS & 1 TEASPOONFUL FOR 70 KG PERSON (150 LB)
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-76
Caustics
Strong alkaline chemicals that destroy soft body tissues resulting in a deep, penetrating type of burn, in contrast to corrosives, that result in a more superficial type of damage via chemical means or inflammation. Caustics are usually hydroxides of light metals. SODIUM HYDROXIDE and potassium hydroxide are the most widely used caustic agents in industry. Medically, they have been used externally to remove diseased or dead tissues and destroy warts and small tumors. The accidental ingestion of products (household and industrial) containing caustic ingredients results in thousands of injuries per year. (See all compounds classified as Caustics.)
Coloring Agents
Chemicals and substances that impart color including soluble dyes and insoluble pigments. They are used in INKS; PAINTS; and as INDICATORS AND REAGENTS. (See all compounds classified as Coloring Agents.)
The acute and subacute toxicities of several Cr(III) and Cr(VI) compounds (chromium(3+) chloride, chromium(3+) nitrate, chromium(3+) sulfate, chromium trioxide, potassium dichromate) were determined in NZC and (CxO) mice injected ip. The distal median lethal doses (> 10 days after treatment) averaged (17.9 + or - 1.8) X 10(-6) g chromium/g body wt regardless of the oxidation state of the Cr compound injected (chromium(3+) sulfate may be an exception), but acute toxicity (3 days) was much greater with Cr(VI) compounds. Acid digests of entire male mice that were administered ip 1/6 of the distal LD50, either once or repeatedly at weekly intervals, were analyzed to determine the whole body persistence and clearance kinetics of Cr. Mice dosed once with Cr(III) retained 6.5 times more chromium at 21 days than mice treated with Cr(VI). When Cr(III) was given at weekly intervals mice accumulated 6 times more Cr by 8 wk than Cr(VI)-treated mice, though only the latter showed symptoms of chromic toxicity. Whole body Cr concentrations continued to rise with further Cr(III) treatments, but slowly declined with Cr(VI). Analyses of fecal and urinary excretion confirmed that most of the urinary Cr excretion from Cr(VI)-treated animals was much faster in both urine and feces than from mice given Cr(III). The differential storage and clearance kinetics of Cr(III) and Cr(VI) compounds may be significant in experimental Cr carcinogenesis studies and in the toxicology of Cr in workers exposed industrially to potentially carcinogenic chromium-containing dusts or aerosols.
PMID:6652867 Bryson WG, Goodall CM; Carcinogenesis 4 (12): 1535-40 (1983)
The interaction of potassium dichromate (Cr(VI)) with bovine serum albumin (BSA) was investigated by fluorescence, synchronous fluorescence, resonance light scattering (RLS), ultraviolet-visible absorption, and circular dichroism (CD) spectroscopies under simulated physiological conditions. The experimental results showed that Cr(VI) could quench the intrinsic fluorescence of BSA following a static quenching process, which indicates the formation of a Cr(VI)-BSA complex. The binding constant (KA) and binding site (n) were measured at different temperatures. The spectroscopic results also revealed that the binding of Cr(VI) to BSA can lead to the loosening of the protein conformation and can change the microenvironment and skeleton of BSA.
PMID:22095857 Zhang P et al; J Biochem Mol Toxicol 26 (2): 54-9 (2012)
Market Place

ABOUT THIS PAGE
28
PharmaCompass offers a list of Potassium Dichromate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Potassium Dichromate manufacturer or Potassium Dichromate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Potassium Dichromate manufacturer or Potassium Dichromate supplier.
PharmaCompass also assists you with knowing the Potassium Dichromate API Price utilized in the formulation of products. Potassium Dichromate API Price is not always fixed or binding as the Potassium Dichromate Price is obtained through a variety of data sources. The Potassium Dichromate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Dichromic acid (H2Cr2O7), dipotassium salt (8CI) manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Dichromic acid (H2Cr2O7), dipotassium salt (8CI), including repackagers and relabelers. The FDA regulates Dichromic acid (H2Cr2O7), dipotassium salt (8CI) manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Dichromic acid (H2Cr2O7), dipotassium salt (8CI) API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Dichromic acid (H2Cr2O7), dipotassium salt (8CI) supplier is an individual or a company that provides Dichromic acid (H2Cr2O7), dipotassium salt (8CI) active pharmaceutical ingredient (API) or Dichromic acid (H2Cr2O7), dipotassium salt (8CI) finished formulations upon request. The Dichromic acid (H2Cr2O7), dipotassium salt (8CI) suppliers may include Dichromic acid (H2Cr2O7), dipotassium salt (8CI) API manufacturers, exporters, distributors and traders.
click here to find a list of Dichromic acid (H2Cr2O7), dipotassium salt (8CI) suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Dichromic acid (H2Cr2O7), dipotassium salt (8CI) DMF (Drug Master File) is a document detailing the whole manufacturing process of Dichromic acid (H2Cr2O7), dipotassium salt (8CI) active pharmaceutical ingredient (API) in detail. Different forms of Dichromic acid (H2Cr2O7), dipotassium salt (8CI) DMFs exist exist since differing nations have different regulations, such as Dichromic acid (H2Cr2O7), dipotassium salt (8CI) USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Dichromic acid (H2Cr2O7), dipotassium salt (8CI) DMF submitted to regulatory agencies in the US is known as a USDMF. Dichromic acid (H2Cr2O7), dipotassium salt (8CI) USDMF includes data on Dichromic acid (H2Cr2O7), dipotassium salt (8CI)'s chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Dichromic acid (H2Cr2O7), dipotassium salt (8CI) USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Dichromic acid (H2Cr2O7), dipotassium salt (8CI) suppliers with USDMF on PharmaCompass.
Dichromic acid (H2Cr2O7), dipotassium salt (8CI) Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Dichromic acid (H2Cr2O7), dipotassium salt (8CI) GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Dichromic acid (H2Cr2O7), dipotassium salt (8CI) GMP manufacturer or Dichromic acid (H2Cr2O7), dipotassium salt (8CI) GMP API supplier for your needs.
A Dichromic acid (H2Cr2O7), dipotassium salt (8CI) CoA (Certificate of Analysis) is a formal document that attests to Dichromic acid (H2Cr2O7), dipotassium salt (8CI)'s compliance with Dichromic acid (H2Cr2O7), dipotassium salt (8CI) specifications and serves as a tool for batch-level quality control.
Dichromic acid (H2Cr2O7), dipotassium salt (8CI) CoA mostly includes findings from lab analyses of a specific batch. For each Dichromic acid (H2Cr2O7), dipotassium salt (8CI) CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Dichromic acid (H2Cr2O7), dipotassium salt (8CI) may be tested according to a variety of international standards, such as European Pharmacopoeia (Dichromic acid (H2Cr2O7), dipotassium salt (8CI) EP), Dichromic acid (H2Cr2O7), dipotassium salt (8CI) JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Dichromic acid (H2Cr2O7), dipotassium salt (8CI) USP).
