
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Daranide
2. Dichlofenamide
3. Diclofenamid
4. Glauconide
1. Diclofenamide
2. 120-97-8
3. 4,5-dichlorobenzene-1,3-disulfonamide
4. Dichlofenamide
5. Dichlorophenamide
6. Daranide
7. Antidrasi
8. Dichlorphenamid
9. Glauconide
10. Glaucol
11. Oratrol
12. 1,3-benzenedisulfonamide, 4,5-dichloro-
13. 4,5-dichloro-m-benzenedisulfonamide
14. Diclofenamida
15. Diclofenamidum
16. 1,3-disulfamyl-4,5-dichlorobenzene
17. Diclofenamidum [inn-latin]
18. 1,3-disulfamoyl-4,5-dichlorobenzene
19. 4,5-dichloro-1,3-disulfamoylbenzene
20. Diclofenamida [inn-spanish]
21. 3,4-dichloro-5-sulfamylbenzenesulfonamide
22. Keveyis
23. 4,5-dichloro-1,3-benzenedisulfonamide
24. Cb 8000
25. M-benzenedisulfonamide, 4,5-dichloro-
26. Diclofenamide [inn]
27. Dichlorphenamide (usp)
28. Dichlorphenamide [usp]
29. Chembl17
30. Diclofenamid
31. Vvj6673mhy
32. Barastonin
33. Dichlorphenamide (diclofenamide)
34. Glajust
35. Glaumid
36. Glafco
37. Chebi:101085
38. 4,5-dichloro-benzene-1,3-disulfonic Acid Diamide
39. Ncgc00016371-01
40. Cas-120-97-8
41. Dasanide
42. Daranide (tn)
43. Hsdb 3267
44. 120d978
45. Dichlorophenamide (dcp)
46. Einecs 204-440-6
47. Unii-vvj6673mhy
48. Brn 2703329
49. Diclorfenamida
50. Diclorfenammide
51. Sulfonamide 4
52. 2pou
53. 4,5-dichloro-m-benzendisulfonamide
54. Keveyis (tn)
55. Prestwick_1071
56. Dichlorophenamide, Dcp
57. Prestwick0_000809
58. Prestwick1_000809
59. Prestwick2_000809
60. Prestwick3_000809
61. Diclofenamide (jan/inn)
62. Dsstox_cid_2922
63. Diclofenamide [jan]
64. Dsstox_rid_76789
65. Dsstox_gsid_22922
66. Bspbio_000677
67. Dichlorphenamide [mi]
68. Mls002154010
69. Diclofenamide [mart.]
70. Schembl112376
71. Spbio_002598
72. Dichlorphenamide [hsdb]
73. Diclofenamide [who-dd]
74. Bpbio1_000745
75. Gtpl6807
76. Dtxsid1022922
77. Bdbm10883
78. Amy7513
79. Dichlorphenamide [usp-rs]
80. Hms1570b19
81. Hms2097b19
82. Hms2236i07
83. Hms3373g04
84. Hms3655h06
85. Hms3714b19
86. Hms3745k19
87. Zinc896918
88. Bcp22591
89. Ex-a1311
90. Hy-b0397
91. Tox21_110401
92. Mfcd00148948
93. S2177
94. 4,5-dichlorobenzene1,3-disulfonamide
95. Dichlorphenamide [orange Book]
96. Akos015899860
97. Ccg-220809
98. Db01144
99. Dichlorphenamide [usp Impurity]
100. 4,5-dicholorobenzene-1,3-disulfonamide
101. Dichlorphenamide [usp Monograph]
102. Ncgc00016371-02
103. Ncgc00016371-04
104. Ncgc00016371-05
105. Ncgc00016371-06
106. Ncgc00016371-08
107. Ac-25007
108. As-63955
109. I7a
110. Smr001233338
111. 4,5-dichloro-1,3-benzenedisulfonamide #
112. Ft-0648264
113. Sw197138-3
114. A21072
115. C07459
116. D00518
117. Sr-01000841825
118. Q3706987
119. Sr-01000841825-2
120. W-108466
121. Brd-k71499074-001-03-8
122. Dichlorphenamide, United States Pharmacopeia (usp) Reference Standard
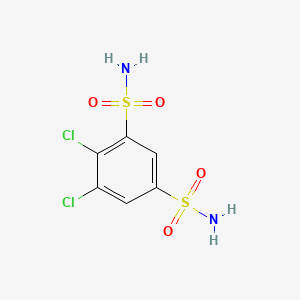
123. Diclofenamide; Dichlofenamide; Daranide; 4,5-dichlorobenzene-1,3-disulfonamide
| Molecular Weight | 305.2 g/mol |
|---|---|
| Molecular Formula | C6H6Cl2N2O4S2 |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 303.9146044 g/mol |
| Monoisotopic Mass | 303.9146044 g/mol |
| Topological Polar Surface Area | 137 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 452 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Carbonic Anhydrase Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
...USED IN TREATMENT OF PRIMARY GLAUCOMA, ACUTE PHASE OF SECONDARY GLAUCOMA, & IN PREOPERATIVE CONTROL OF INTRAOCULAR TENSION. ... ALTHOUGH IT HAS DIURETIC PROPERTIES, IT IS NOT PROMOTED FOR THIS PURPOSE.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 866
...DRUG HAS BEEN FOUND TO INHIBIT EPILEPTIC SEIZURES & TO DECR RATE OF SPINAL FLUID FORMATION. /ACETAZOLAMIDE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 827
...REDUCES RATE OF AQ HUMOR FORMATION; INTRAOCULAR PRESSURE IN PT WITH GLAUCOMA IS CORRESPONDINGLY REDUCED. THIS ACTION OF DRUG APPEARS TO BE INDEPENDENT OF SYSTEMIC ACID-BASE BALANCE. /ACETAZOLAMIDE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 827
For more Therapeutic Uses (Complete) data for DICHLORPHENAMIDE (11 total), please visit the HSDB record page.
IT HAS BEEN SUGGESTED THAT POSTOPERATIVE USE /AFTER IRIDECTOMY/...MAY ADVERSELY AFFECT OUTCOME OF FILTERING OPERATIONS BY REDUCING SIZE OF RESULTANT DRAINAGE BLEB & DELAYING REFORMATION OF ANTERIOR CHAMBER. /CARBONIC ANHYDRASE INHIBITORS/
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 933
...SHOULD BE USED CAUTIOUSLY IN PT WITH OBSTRUCTIVE PULMONARY DISEASE BECAUSE THEY MAY PPT ACUTE RESPIRATORY FAILURE. /CARBONIC ANHYDRASE INHIBITORS/
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 933
DIURESIS MAY BE TROUBLESOME INITIALLY BUT SUBSIDES DURING CONTINUED THERAPY BECAUSE OF PERSISTENT METABOLIC ACIDOSIS. /CARBONIC ANHYDRASE INHIBITORS/
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 932
...BE GIVEN CAUTIOUSLY TO PT...WITH DISEASES ASSOC WITH INCR MINERALOCORTICOID ACTIVITY (EG, PRIMARY HYPERALDOSTERONISM, CUSHING'S SYNDROME) & THOSE RECEIVING POTASSIUM-WASTING DRUGS (EG, THIAZIDES, LOOP DIURETICS, CORTICOSTEROIDS). /CARBONIC ANHYDRASE INHIBITORS/
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 933
For more Drug Warnings (Complete) data for DICHLORPHENAMIDE (7 total), please visit the HSDB record page.
For adjunctive treatment of: chronic simple (open-angle) glaucoma, secondary glaucoma, and preoperatively in acute angle-closure glaucoma where delay of surgery is desired in order to lower intraocular pressure
Dichlorphenamide is an oral carbonic anhydrase inhibitor indicated for adjunctive treatment of: chronic simple (open-angle) glaucoma, secondary glaucoma, and preoperatively in acute angle-closure glaucoma where delay of surgery is desired in order to lower intraocular pressure. Carbonic anhydrase inhibitors reduce intraocular pressure by partially suppressing the secretion of aqueous humor (inflow).
Carbonic Anhydrase Inhibitors
A class of compounds that reduces the secretion of H+ ions by the proximal kidney tubule through inhibition of CARBONIC ANHYDRASES. (See all compounds classified as Carbonic Anhydrase Inhibitors.)
S - Sensory organs
S01 - Ophthalmologicals
S01E - Antiglaucoma preparations and miotics
S01EC - Carbonic anhydrase inhibitors
S01EC02 - Diclofenamide
Carbonic anhydrase inhibitors reduce intraocular pressure by partially suppressing the secretion of aqueous humor (inflow), although the mechanism by which they do this is not fully understood. Evidence suggests that HCO3- ions are produced in the ciliary body by hydration of carbon dioxide under the influence of carbonic anhydrase and diffuse into the posterior chamber which contains more Na+ and HCO3- ions than does plasma and consequently is hypertonic. Water is then attracted to the posterior chamber by osmosis, resulting in a drop in pressure.
PHOSPHATURIA MAY BE RELATED TO DIRECT STIMULATION...OF CYCLIC ADENOSINE 3',5'-MONOPHOSPHATE...PRODN BY KIDNEY. ...DRUG ACTS SIMILARLY TO PARATHYROID HORMONE IN ENHANCING URINARY EXCRETION OF PHOSPHATE & CYCLIC AMP, IN CONTRAST TO ITS ANTAGONISM OF ACTION OF HORMONE ON BONE. /ACETAZOLAMIDE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 826
...INHIBITION OF...CARBONIC ANHYDRASE. ...IS NONCOMPETITIVE. ...ENZYME IS NORMALLY PRESENT IN TISSUES IN HUGE EXCESS. MORE THAN 99% OF ENZYME ACTIVITY IN KIDNEY MUST BE INHIBITED BEFORE PHYSIOLOGICAL EFFECTS BECOME APPARENT. ENZYME...IS DOMINANT TISSUE COMPONENT TO WHICH INHIBITORS BECOME BOUND. /ACETAZOLAMIDE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 826
/CHANGES IN URINE/...MAY BE ATTRIBUTED TO INHIBITION OF (+)H SECRETION BY RENAL TUBULE. ... CURRENT EVIDENCE INDICATES GREATER EFFECT ON PROXIMAL THAN ON DISTAL TUBULE, WITH LITTLE OR NO EFFECT ON ASCENDING LIMB. ...PHOSPHATURIA...USED AS INDEX OF LOCALIZING DIURETIC ACTION... /ACETAZOLAMIDE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 826
...INCR URINARY EXCRETION OF BICARBONATE & FIXED CATION, MOSTLY SODIUM. AS RESULT, CONCN OF BICARBONATE IN EXTRACELLULAR FLUID DECR & METABOLIC ACIDOSIS RESULTS. ...RENAL RESPONSE TO ACETAZOLAMIDE IS GREATLY REDUCED.../BUT/ DIURETIC RESPONSE IS ENHANCED. /ACETAZOLAMIDE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 826
DRUG LOWERS INTRAOCULAR PRESSURE BY REDUCING RATE OF SECRETION OF AQ HUMOR.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 867

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
42
PharmaCompass offers a list of Dichlorphenamide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Dichlorphenamide manufacturer or Dichlorphenamide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Dichlorphenamide manufacturer or Dichlorphenamide supplier.
PharmaCompass also assists you with knowing the Dichlorphenamide API Price utilized in the formulation of products. Dichlorphenamide API Price is not always fixed or binding as the Dichlorphenamide Price is obtained through a variety of data sources. The Dichlorphenamide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Diclofenamide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Diclofenamide, including repackagers and relabelers. The FDA regulates Diclofenamide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Diclofenamide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Diclofenamide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Diclofenamide supplier is an individual or a company that provides Diclofenamide active pharmaceutical ingredient (API) or Diclofenamide finished formulations upon request. The Diclofenamide suppliers may include Diclofenamide API manufacturers, exporters, distributors and traders.
click here to find a list of Diclofenamide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Diclofenamide DMF (Drug Master File) is a document detailing the whole manufacturing process of Diclofenamide active pharmaceutical ingredient (API) in detail. Different forms of Diclofenamide DMFs exist exist since differing nations have different regulations, such as Diclofenamide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Diclofenamide DMF submitted to regulatory agencies in the US is known as a USDMF. Diclofenamide USDMF includes data on Diclofenamide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Diclofenamide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Diclofenamide suppliers with USDMF on PharmaCompass.
A Diclofenamide written confirmation (Diclofenamide WC) is an official document issued by a regulatory agency to a Diclofenamide manufacturer, verifying that the manufacturing facility of a Diclofenamide active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Diclofenamide APIs or Diclofenamide finished pharmaceutical products to another nation, regulatory agencies frequently require a Diclofenamide WC (written confirmation) as part of the regulatory process.
click here to find a list of Diclofenamide suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Diclofenamide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Diclofenamide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Diclofenamide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Diclofenamide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Diclofenamide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Diclofenamide suppliers with NDC on PharmaCompass.
Diclofenamide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Diclofenamide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Diclofenamide GMP manufacturer or Diclofenamide GMP API supplier for your needs.
A Diclofenamide CoA (Certificate of Analysis) is a formal document that attests to Diclofenamide's compliance with Diclofenamide specifications and serves as a tool for batch-level quality control.
Diclofenamide CoA mostly includes findings from lab analyses of a specific batch. For each Diclofenamide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Diclofenamide may be tested according to a variety of international standards, such as European Pharmacopoeia (Diclofenamide EP), Diclofenamide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Diclofenamide USP).
