
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
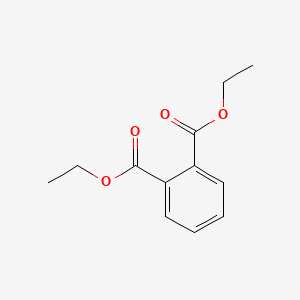

1. Phthalic Acid Diethyl Ester
1. 84-66-2
2. Ethyl Phthalate
3. Phthalic Acid Diethyl Ester
4. Anozol
5. Diethylphthalate
6. Diethyl Benzene-1,2-dicarboxylate
7. Neantine
8. Phthalol
9. Solvanol
10. Diethyl O-phthalate
11. Palatinol A
12. Placidol E
13. Unimoll Da
14. Phthalsaeurediaethylester
15. O-bis(ethoxycarbonyl)benzene
16. Diethyl 1,2-benzenedicarboxylate
17. 1,2-diethyl Phthalate
18. 1,2-benzenedicarboxylic Acid, Diethyl Ester
19. Estol 1550
20. O-benzenedicarboxylic Acid Diethyl Ester
21. Di-n-ethyl Phthalate
22. Diethyl O-phenylenediacetate
23. Diethyl Phtalate
24. Phthalic Acid, Diethyl Ester
25. Rcra Waste Number U088
26. Diethylester Kyseliny Ftalove
27. Nci-c60048
28. 1,2-benzenedicarboxylic Acid Diethyl Ester
29. Nsc 8905
30. Chebi:34698
31. Diethyl Phthalate (nf)
32. Diethyl Phthalate [nf]
33. Nsc-8905
34. 1,2-benzenedicarboxylic Acid, 1,2-diethyl Ester
35. O-phthalic Acid, Diethyl Ester
36. Uf064m00af
37. 1,2-benzenedicarboxylic Acid, Di-c4-13-alkyl Esters
38. Ncgc00090974-03
39. O-benzenedicarboxylic Acid, Diethyl Ester
40. Diethyl Phthalate, 99%
41. Dsstox_cid_1780
42. Dsstox_rid_76323
43. Dsstox_gsid_21780
44. Phthalic Acid, Bis-iso-nonyl Ester
45. Diethyl-phthalate
46. Cas-84-66-2
47. Smr000857334
48. Ccris 2675
49. Hsdb 926
50. Dpx-f5384
51. Phthalsaeurediaethylester [german]
52. Einecs 201-550-6
53. Diethyl Phthalate, Pestanal(r), Analytical Standard
54. Diethylester Kyseliny Ftalove [czech]
55. Rcra Waste No. U088
56. Brn 1912500
57. Unii-uf064m00af
58. Ai3-00329
59. Kodaflex Dep
60. Diethyl-o-phthalate
61. Diethyl Phthalic Acid
62. Phthalic Acid Diethyl
63. Diethyl Phthalate, Nf
64. Diethyl 1,2-benzenedioate
65. Bmse000846
66. Epitope Id:140105
67. Ec 201-550-6
68. Wln: 2ovr Bvo2
69. Diethyl Phthalate, >=99%
70. Diethyl Phthalate, 99.5%
71. Schembl22296
72. Dimethyphalate ,ethylphthalate
73. 4-09-00-03172 (beilstein Handbook Reference)
74. Mls001336021
75. Mls001336022
76. Mls002152901
77. Mls002177800
78. Bidd:er0639
79. Diethyl Phthalate [ii]
80. Phthalic Acid, Bis-ethyl Ester
81. Chembl388558
82. Zinc1287
83. Diethyl Phthalate, >=99.5%
84. Diethyl Phthalate [hsdb]
85. Diethyl Phthalate [inci]
86. Dtxsid7021780
87. Diethyl-1,2-benzenedicarboxylate
88. Phthalic Acid Ethyl Ester
89. Diethyl Phthalate [vandf]
90. Ethyl Phthalate [who-dd]
91. Nsc8905
92. Diethyl Phthalate [mart.]
93. Diethyl Phthalate, Lr, >=99%
94. Hms2233j05
95. Hms3369g01
96. Phthalic Acid,diethyl Ester
97. Diethyl Phthalate [usp-rs]
98. Diethyl Phthalate/dimethyl Phthalate
99. Str04116
100. Tox21_111050
101. Tox21_201874
102. Tox21_300183
103. Bbl011577
104. Mfcd00009111
105. Stl163320
106. Akos000119867
107. Phthalic Acid Ethyl Ester [mi]
108. 1,2-diethyl Benzene-1,2-dicarboxylate
109. Diethyl Phthalate [ep Monograph]
110. Diethyl Phthalate Mil-d-242 Mil Spec
111. Ncgc00090974-01
112. Ncgc00090974-02
113. Ncgc00090974-04
114. Ncgc00090974-05
115. Ncgc00090974-06
116. Ncgc00254098-01
117. Ncgc00259423-01
118. 68988-18-1
119. Diethyl Phthalate Metal Plastic Ibc/tote
120. Benzene-1,2-dicarboxylic Acid Diethyl Ester
121. Cs-0013981
122. Ft-0624802
123. Ft-0666787
124. P0296
125. Diethyl Ester Of 1,2-benzenedicarboxylic Acid
126. Diethylphthalate, A-a-59314, Jan-d-242
127. D03804
128. Diethyl Phthalate, Saj Special Grade, >=98.0%
129. Q419811
130. Q-200982
131. F1908-0104
132. Phthalic Acid, Bis-ethyl Ester 100 Microg/ml In Methanol
133. Phthalic Acid, Bis-ethyl Ester 1000 Microg/ml In Methanol
134. Phthalic Acid, Bis-ethyl Ester 100 Microg/ml In Acetonitrile
135. Diethyl Phthalate, European Pharmacopoeia (ep) Reference Standard
136. Diethyl Phthalate, United States Pharmacopeia (usp) Reference Standard
137. Diethyl Phthalate, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 222.24 g/mol |
|---|---|
| Molecular Formula | C12H14O4 |
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | 222.08920892 g/mol |
| Monoisotopic Mass | 222.08920892 g/mol |
| Topological Polar Surface Area | 52.6 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 223 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Absorption of diethyl phthalate and three other phthalates (dimethyl, dibutyl, and di(2-ethylhexyl)) was measured using human epidermal skin obtained from the abdominal skin of 11 cadavers (mostly females 55 years of age or older) and subcutaneous fat removed in vitro. Epidermal membranes were set up in glass diffusion cells, and their permeability to tritiated water was measured to establish the integrity of the skin. Lag time for absorption of diethyl phthalate was 6 hr, and the steady-state absorption rate was 12.8 ug/sq cm per hour.
International Programme on Chemical Safety (IPCS); Concise International Chemical Assessment Document (CICADS) 52: Diethyl Phthalate (2003) Available from, as of April 17, 2008: https://www.inchem.org/documents/cicads/cicads/cicad52.htm
Percutaneous absorption of diethyl phthalate was evaluated in vitro in flow-through diffusion cells using human breast skin. Neat chemical (16-21 mg/sq cm) was applied over 72 hr to the epidermal surface of the skin, which was either uncovered or covered. The absorption of diethyl phthalate through skin was 3.9% and 4.8% of the applied doses for covered and uncovered conditions, respectively. The interindividual variation was 4-fold, ranging from 1.6% (SD 1.2) (n = 3) to 8.7% (SD 3.9) (n = 6) among skin donors.
International Programme on Chemical Safety (IPCS); Concise International Chemical Assessment Document (CICADS) 52: Diethyl Phthalate (2003) Available from, as of April 17, 2008: https://www.inchem.org/documents/cicads/cicads/cicad52.htm
This study examined the extent of dermal absorption of a series of phthalate diesters in the rat. Those tested were dimethyl, diethyl, dibutyl, diisobutyl, dihexyl, di(2-ethylhexyl), diisodecyl, and benzyl butyl phthalate. Hair from a skin area (1.3 cm in diameter) on the back of male F344 rats was clipped, the 14(C)phthalate diester was applied in a dose of 157 mumol/kg, and the area of application was covered with a perforated cap. The rat was restrained and housed for 7 days in a metabolic cage that allowed separate collection of urine and feces. Urine and feces were collected every 24 hr, and the amount of (14)C excreted was taken as an index of the percutaneous absorption. At 24 hr, diethyl phthalate showed the greatest excretion (26%). As the length of the alkyl side chain increased, the amount of (14)C excreted in the first 24 hr decreased signficantly. The cumulative percentage dose excreted in 7 days was greatest for diethyl, dibutyl, and diisobutyl phthalate, about 50-60% of the applied (14)C; and intermediate (20-40%) for dimethyl, benzyl butyl, and dihexyl phthalate. Urine was the major route of excretion of all phthalate diesters except for diisodecyl phthalate. This compound was poorly absorbed and showed almost no urinary excretion. After 7 days, the percentage dose for each phthalate that remained in the body was minimal showed no specific tissue distribution. Most of the unexcreted dose remained in the area of application. These data show that the structure of the phthalate diester determines the degree of dermal absorption. Absorption maximized with diethyl phthalate and then decreased significantly as the alkyl side chain length increased.
PMID:2925020 Elsisi AE et al; Fundam Appl Toxicol 12 (1): 70-7 (1989)
(14)C-Carboxy-labelled diethyl phthalate (2850 mg/kg body weight) was administered intraperitoneally to a group of 13 pregnant rats on either day 5 or day 10 of gestation. The results showed that radioactivity in the maternal blood peaked during the first 24 hr, then diminished quickly. A similar pattern was observed in amniotic fluid and fetal tissues. The reduction in concentration of (14)C from these tissues as a function of time was found to fit a first-order excretion curve. From this model curve, the half-life was calculated to be 2.22 days for diethyl phthalate. Radioactivity from (14)C-diethyl phthalate is transmitted across the placenta from mother to fetus for at least 15 days post-injection. (14)C radioactivity was widely distributed and was detected (<1%) in maternal blood, placenta, amniotic fluid, and developing fetuses at all gestational stages investigated.
International Programme on Chemical Safety (IPCS); Concise International Chemical Assessment Document (CICADS) 52: Diethyl Phthalate (2003) Available from, as of April 17, 2008: https://www.inchem.org/documents/cicads/cicads/cicad52.htm
For more Absorption, Distribution and Excretion (Complete) data for DIETHYL PHTHALATE (12 total), please visit the HSDB record page.
Diethyl phthalate (10 or 100 mg) was administered to each of three Wistar rats by stomach intubation. Daily urine collections were analyzed for 10 days by GC-MS. For both doses, 77-78% of the administered dose was excreted in urine within 24 hr as monoester derivative (67-70% of the dose), phthalic acid (8-9% of the dose), or parent compound (0.1-0.4%), and about 85-93% was excreted within 1 week after administration.
International Programme on Chemical Safety (IPCS); Concise International Chemical Assessment Document (CICADS) 52: Diethyl Phthalate (2003) Available from, as of April 17, 2008: https://www.inchem.org/documents/cicads/cicads/cicad52.htm
Using in vitro preparations of rat, baboon, and human tissues, /investigators/ demonstrated that DEP was hydrolyzed to the monoester. Further hydrolysis of the monoester and its conjugation with glucuronic acid are consistent with the biotransformation pathways for related phthalates.
American Conference of Governmental Industrial Hygienists. Documentation of the TLV's and BEI's with Other World Wide Occupational Exposure Values. CD-ROM Cincinnati, OH 45240-1634 2007.
The first step of metabolism involves hydrolysis to a monoester derivative. This was seen in the in vitro metabolism of (14)C-diethyl phthalate (5-mmol/L solution) by hepatic and small intestine preparations from a rodent (rat), a nonrodent (ferret), and a nonhuman primate (baboon). Hepatic postmitochondrial supematant and intestinal preparations from the rat, baboon, and ferret were able to catalyze the hydrolysis of diethyl phthalate to its monoester derivative. Enzyme activity was expressed as micromoles of product formed per hour per gram of liver (umol/hour/g) or per milligram of intestinal mucosal cell protein (umol/hour/mg). Quantitative species differences were observed in the hepatic and intestinal studies. In the hepatic studies, diethyl phthalate hydrolase activity decreased in the following order: baboon (516 umol/hour/g) > rat (231 umol/hour/g) > ferret (45.9 umol/hour/g). In the intestinal preparation, diethyl phthalate hydrolase activity decreased in the same order: baboon (4.33 umol/hour/mg) > rat (0.648 umol/hour/mg) > ferret (0.053 umol/hour/mg). Studies were also performed with samples of human duodenum and jejunum tissues. As with the three animal species, human intestinal preparations were also active in the metabolism of diethyl phthalate. The results obtained with human intestinal preparations were expressed as nanomoles of product formed per hour per milligram of intestinal protein (nmol/hour/mg). In the human intestinal preparation, the diethyl phthalate hydrolase activity was 31.2-153 nmol/hour/mg in the duodenum and 129 nmol/hour/mg in the jejunum. Similarly, of the tissues from three rat and one human studied in vitro, the rat small intestine hydrolyzed the greatest amount (36.4%) of diethyl phthalate in a 16-hour period. These results show a qualitative species similarity in the hydrolytic metabolism of diethyl phthalate in humans, a rodent, a nonrodent, and a nonhuman primate.
U.S. Dept Health & Human Services/Agency for Toxic Substances & Disease Registry; Toxicological Profile for Diethylphthalate p.34-5 (1995) PB/95/264214/AS. Available from, as of April 23, 2008: https://www.atsdr.cdc.gov/toxpro2.html#
Once formed, the monoester derivative can be further hydrolysed in vivo to phthalic acid and excreted or conjugated to glucuronide and excreted; the terminal or next-to-last carbon atom in the monoester can be oxidized to an alcohol and excreted; or the alcohol can be successively oxidized to an aldehyde, ketone, or carboxylic acid and excreted.
International Programme on Chemical Safety (IPCS); Concise International Chemical Assessment Document (CICADS) 52: Diethyl Phthalate (2003) Available from, as of April 17, 2008: https://www.inchem.org/documents/cicads/cicads/cicad52.htm
For more Metabolism/Metabolites (Complete) data for DIETHYL PHTHALATE (8 total), please visit the HSDB record page.
(14)C-Carboxy-labelled diethyl phthalate (2850 mg/kg body weight) was administered intraperitoneally to a group of 13 pregnant rats on either day 5 or day 10 of gestation. ... The half-life was calculated to be 2.22 days for diethyl phthalate.
International Programme on Chemical Safety (IPCS); Concise International Chemical Assessment Document (CICADS) 52: Diethyl Phthalate (2003) Available from, as of April 17, 2008: https://www.inchem.org/documents/cicads/cicads/cicad52.htm
In fish, the half-life may be as short as 1.5 hr, yielding 99% clearance in 24 hr. /Phthalate esters/
Nat'l Research Council Canada; Phthalate Esters p.20 (1980) NRCC No. 17583
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 11846
Submission : 1996-02-05
Status : Inactive
Type : II


 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
40
PharmaCompass offers a list of Diethyl Phthalate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Diethyl Phthalate manufacturer or Diethyl Phthalate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Diethyl Phthalate manufacturer or Diethyl Phthalate supplier.
PharmaCompass also assists you with knowing the Diethyl Phthalate API Price utilized in the formulation of products. Diethyl Phthalate API Price is not always fixed or binding as the Diethyl Phthalate Price is obtained through a variety of data sources. The Diethyl Phthalate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Diethyl Phthalate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Diethyl Phthalate, including repackagers and relabelers. The FDA regulates Diethyl Phthalate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Diethyl Phthalate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Diethyl Phthalate supplier is an individual or a company that provides Diethyl Phthalate active pharmaceutical ingredient (API) or Diethyl Phthalate finished formulations upon request. The Diethyl Phthalate suppliers may include Diethyl Phthalate API manufacturers, exporters, distributors and traders.
click here to find a list of Diethyl Phthalate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Diethyl Phthalate DMF (Drug Master File) is a document detailing the whole manufacturing process of Diethyl Phthalate active pharmaceutical ingredient (API) in detail. Different forms of Diethyl Phthalate DMFs exist exist since differing nations have different regulations, such as Diethyl Phthalate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Diethyl Phthalate DMF submitted to regulatory agencies in the US is known as a USDMF. Diethyl Phthalate USDMF includes data on Diethyl Phthalate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Diethyl Phthalate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Diethyl Phthalate suppliers with USDMF on PharmaCompass.
Diethyl Phthalate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Diethyl Phthalate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Diethyl Phthalate GMP manufacturer or Diethyl Phthalate GMP API supplier for your needs.
A Diethyl Phthalate CoA (Certificate of Analysis) is a formal document that attests to Diethyl Phthalate's compliance with Diethyl Phthalate specifications and serves as a tool for batch-level quality control.
Diethyl Phthalate CoA mostly includes findings from lab analyses of a specific batch. For each Diethyl Phthalate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Diethyl Phthalate may be tested according to a variety of international standards, such as European Pharmacopoeia (Diethyl Phthalate EP), Diethyl Phthalate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Diethyl Phthalate USP).

