
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
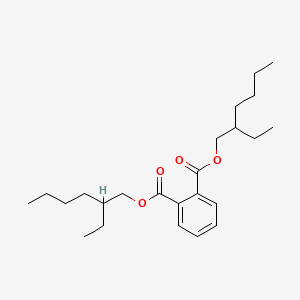

1. Bis(2-ethylhexyl)phthalate
2. Dehp
3. Di 2 Ethylhexylphthalate
4. Di(2-ethylhexyl)phthalate
5. Di-2-ethylhexylphthalate
6. Diethylhexyl Phthalate
7. Dioctyl Phthalate
8. Phthalate, Diethylhexyl
9. Phthalate, Dioctyl
1. Dehp
2. 117-81-7
3. Di(2-ethylhexyl)phthalate
4. Di(2-ethylhexyl) Phthalate
5. Bis(2-ethylhexyl)phthalate
6. Diethylhexyl Phthalate
7. 2-ethylhexyl Phthalate
8. Di-sec-octyl Phthalate
9. Octyl Phthalate
10. Fleximel
11. Octoil
12. Ethylhexyl Phthalate
13. Palatinol Ah
14. Vestinol Ah
15. Bisoflex Dop
16. Di-2-ethylhexyl Phthalate
17. Kodaflex Dop
18. Staflex Dop
19. Truflex Dop
20. Flexol Dop
21. Vinicizer 80
22. Bisoflex 81
23. Eviplast 80
24. Eviplast 81
25. Hercoflex 260
26. Rc Plasticizer Dop
27. Compound 889
28. Witcizer 312
29. Platinol Dop
30. Nuoplaz Dop
31. Platinol Ah
32. Hatcol Dop
33. Reomol Dop
34. Pittsburgh Px-138
35. Sansocizer Dop
36. Ergoplast Fdo
37. Monocizer Dop
38. Plasthall Dop
39. Flexol Plasticizer Dop
40. Mollan O
41. Jayflex Dop
42. Sicol 150
43. Ergoplast Fdo-s
44. Di(2-ethylhexyl)orthophthalate
45. Dioctylphthalate
46. Good-rite Gp 264
47. Reomol D 79p
48. Bis(2-ethylhexyl) Benzene-1,2-dicarboxylate
49. Di(ethylhexyl) Phthalate
50. Bis(ethylhexyl) Phthalate
51. Rcra Waste Number U028
52. Phthalic Acid Dioctyl Ester
53. Nci-c52733
54. Di(2-ethylhexyl) O-phthalate
55. Phthalic Acid Di(2-ethylhexyl) Ester
56. 1,2-benzenedicarboxylic Acid, Bis(2-ethylhexyl) Ester
57. Dop
58. Bis(2-ethylhexyl) 1,2-benzenedicarboxylate
59. Bis(2-ethylhexyl) O-phthalate
60. Phthalic Acid, Bis(2-ethylhexyl) Ester
61. Chebi:17747
62. 1,2-benzenedicarboxylic Acid Bis(2-ethylhexyl) Ester
63. Benzenedicarboxylic Acid, Bis(2-ethylhexyl) Ester
64. Phthalic Acid Bis(2-ethylhexyl) Ester
65. Bis-(2-ethylhexyl)ester Kyseliny Ftalove
66. C42k0ph13c
67. Dtxsid5020607
68. Etalon
69. Bis-(2-ethylhexyl)ester Kyseliny Ftalove [czech]
70. Di-(2-ethylhexyl) Phthalate
71. Bis-(2-ethylhexyl) Phthalate
72. Nsc-17069
73. Phthalic Acid Bis(2-ethylhexyl Ester)
74. Ncgc00091499-05
75. 1,2-benzenedicarboxylic Acid, 1,2-bis(2-ethylhexyl) Ester
76. Sconamoll Dop
77. Diacizer Dop
78. Kodaflex Dehp
79. 15495-94-0
80. Etalon (plasticizer)
81. Sansocizer R 8000
82. Caswell No. 392k
83. Behp
84. Di-2-ethylhexylphthalate
85. Diplast O; Esbo-d 82; Ergoplast Fdo; Ergoplast Fdo-s; Etalon
86. Phthalic Acid, Bis-2-ethylhexyl Ester
87. Dof [russian Plasticizer]
88. Smr000777878
89. Ccris 237
90. Ethyl Hexyl Phthalate
91. Hsdb 339
92. Di(2-ethylhexyl) Orthophthalate
93. Bis-(2-ethylhexyl)ester Kyseliny Ftalove (czech)
94. Einecs 204-211-0
95. Nsc 17069
96. Diethylhexylphthalate (bis-(2-ethylhexyl) Phthalate)
97. Rcra Waste No. U028
98. Union Carbide Flexol 380
99. Epa Pesticide Chemical Code 295200
100. Brn 1890696
101. Unii-c42k0ph13c
102. Ai3-04273
103. Daf 68
104. Palatinol Dop
105. Polycizer Dop
106. Merrol Dop
107. Palatinol Ah-l
108. Hatco Dop
109. Vinycizer 80
110. Di(2-ethylhexyl)phthalate (dehp)
111. Mfcd00009493
112. Corflex 400
113. 8033-53-2
114. Dioctyl Phthalate, 99%
115. Dsstox_cid_607
116. 1, Bis(ethylhexyl) Ester
117. Dehp [mi]
118. Epitope Id:140107
119. Ec 204-211-0
120. Wln: 8ovr Bvo8
121. Di(2-ethylhexyl Phthalate)
122. Dsstox_rid_75688
123. Plastic Additive 14
124. Dsstox_gsid_20607
125. Schembl20271
126. Dioctylphthalate [ii]
127. 14c -dehp
128. 50885-87-5
129. Mls001333173
130. Mls001333174
131. Mls002454397
132. Dioctyl Phthalate, >=99.5%
133. 1,2-benzenedicarboxylic Acid, Bis-(1-ethylhexyl) Ester
134. Chembl1242017
135. Schembl21733281
136. Hms2233c15
137. Hms3374j09
138. Amy40790
139. Hy-b1945
140. Nsc17069
141. Tox21_400084
142. Bis(2-ethylhexyl)ester Phthalic Acid
143. Diethylhexyl Phthalate [inci]
144. S3360
145. Bis(2-ethylhexyl Phthalate)-
146. Akos024318875
147. Bis(2-ethylhexyl) Phthalate-[13c6]
148. Plastic Additive 14 [usp-rs]
149. Ncgc00091499-01
150. Ncgc00091499-02
151. Ncgc00091499-04
152. Ncgc00091499-06
153. Ncgc00091499-07
154. Cas-117-81-7
155. Di(2-ethylhexyl)phthalate [iarc]
156. Bis(2-ethylhexyl)phthalate [hsdb]
157. Bis(2-ethylhexyl) 1, 2-benzenedicarboxylate
158. Cs-0014050
159. Ft-0624576
160. Ft-0663286
161. P0297
162. Wln: 4y2 & 1ovr Bvo1y4 & 2
163. Bis(2-ethylhexyl) Phthalate, Selectophore(tm)
164. C03690
165. A937603
166. Q418492
167. 1,2-benzenedicarboxylic Acid Bis-(1-ethylhexyl) Ester
168. Benzene-1,2-dicarboxylic Acid Bis(2-ethylhexyl) Ester
169. Brd-a89471977-001-05-2
170. Bis(2-ethylhexyl) Phthalate 100 Microg/ml In Methanol
171. Bis(2-ethylhexyl) Phthalate 5000 Microg/ml In Methanol
172. F0001-0292
173. Bis(2-ethylhexyl) Phthalate, Saj First Grade, >=98.0%
174. Bis(2-ethylhexyl) Phthalate, Pestanal(r), Analytical Standard
175. Phthalic Acid, Bis-2-ethylhexyl Ester 10 Microg/ml In Cyclohexane
176. Phthalic Acid, Bis-2-ethylhexyl Ester 100 Microg/ml In Acetonitrile
177. Phthalic Acid, Bis-2-ethylhexyl Ester 1000 Microg/ml In Cychohexane
178. Plastic Additive 01, European Pharmacopoeia (ep) Reference Standard
179. Bis(2-ethylhexyl) Phthalate, Certified Reference Material, Tracecert(r)
180. Plastic Additive 14, United States Pharmacopeia (usp) Reference Standard
181. 1,2-benzenedicarboxylic Acid, Bis(2-ethylhexyl) Ester, Labeled With Carbon-14
182. 82208-43-3
| Molecular Weight | 390.6 g/mol |
|---|---|
| Molecular Formula | C24H38O4 |
| XLogP3 | 7.4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 16 |
| Exact Mass | 390.27700969 g/mol |
| Monoisotopic Mass | 390.27700969 g/mol |
| Topological Polar Surface Area | 52.6 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 394 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Plasticizers
Materials incorporated mechanically in plastics (usually PVC) to increase flexibility, workability or distensibility; due to the non-chemical inclusion, plasticizers leach out from the plastic and are found in body fluids and the general environment. (See all compounds classified as Plasticizers.)
When administered either IV or orally ... it is mainly excreted in urine and bile.
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-204
It appeared to be rapidly cleared from blood, most being removed within 5-7 hr of completion of dialysis.
PMID:647906 LEWIS LM ET AL; CLIN CHEM 24 (5): 741 (1978)
In one study of subjects who received hemodialysis, blood transfusions or blood that had previously been in contact with polyvinyl chloride medical products, di(2-ethylhexyl) phthalate was found at the following levels (ug/g wet tissue): brain (1.9), heart (0.5), kidney (1.2-2.2), liver (1.5-4.6), lung (1.4-2.2) & spleen (2.2-4.7).
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V29 278 (1982)
The levels of di(2-ethylhexyl) phthalate in neonatal heart tissue from infants who had undergone umbilical catheterization, either alone or with admin of blood products, were reported to be higher than those in similar tissue from untreated infants.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V29 278 (1982)
For more Absorption, Distribution and Excretion (Complete) data for BIS(2-ETHYLHEXYL) PHTHALATE (67 total), please visit the HSDB record page.
It is hypothesized that the teratogen di(2-ethylhexyl) phthalate (DEHP) acts by in vivo hydrolysis to 2-ethylhexanol (2-EXHO), which in turn is metabolized to 2-ethylhexanoic acid (2-EXHA), the proximate teratogen. Teratological studies were conducted with Wistar rats, with administration of these agents on day 12 of geatation. On an equimolar basis DEHP was least potent, 2-ethylhexanol was intermediate, and 2-ethylhexanoic acid was the most potent of the three agents, which is consistent with the hypothesis. Similarity in the types of defects found with these agents also suggests a common mechanism, with 2-ethylhexanoic acid as the proximate teratogen.
PMID:3105103 Ritter EJ; Teratology 35 (1): 41-6 (1987)
When admin either iv or orally, it is rapidly metabolized to derivatives of mono-(2-ethylhexyl)-phthalate. ...
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-204
Rats have been reported to metabolize di(2-ethylhexyl) phthalate to 5-keto-2-ethylhexyl phthalate, 5-carboxyl-2-ethylpentyl phthalate, 5-hydroxy-2-ethylhexyl phthalate & 2-carboxymethylbutyl phthalate after initial hydrolysis to mono(2-ethylhexyl) phthalate.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V29 283 (1982)
African green monkeys & ferrets, in contrast to rats, excrete di(2-ethylhexyl) phthalate metabolites in urine as glucuronide derivatives of mono(2-ethylhexyl) phthalate. Glucuronidation appears to occur at the free carboxyl group, while 2-ethylhexyl substituent is oxidized to an alcohol.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V29 283 (1982)
For more Metabolism/Metabolites (Complete) data for BIS(2-ETHYLHEXYL) PHTHALATE (41 total), please visit the HSDB record page.
The levels of DEHP and MEHP in plasma have been studied in newborn infants given blood exchange transfusions. In one case the MEHP half-life was the same as for DEHP (about 12 hr), indicating that the hydrolysis of DEHP was the rate-limiting metabolic step. However, in other children the half-time of MEHP was longer than that of DEHP ... .
WHO; Environ Health Criteria 131: Diethylhexyl Phthalate p.55 (1992)
After the iv admin of radiolabelled DEHP, at least two elimination phases of radioactivity, with short half-lives (4.5-9 and 22 min, respectively), were observed in rat blood ... After 7 wk of oral admin, the elimination phase in the liver was considerably slower, the half-life being 3-5 days ... No accumulation of DEHP or MEHP was observed when the dosage was 2.8 g/kg/day for 7 days ... nor was there any in a long-term (5-7 weeks) feeding study at a dose level of 1 or 5 g/kg diet (corresponding to a daily dose of about 50 and 250 mg/kg bw) ... .
WHO; Environ Health Criteria 131: Diethylhexyl Phthalate p.56 (1992)
... The mean plasma elimination halflives of MEHP were 3.9, 3.1 and 2.8 hours, respectively for 25, 40 and 60 days old /Sprague-Dawley/ rats. ...
European Chemicals Bureau; European Union Risk Assessment Report, Bis(2-ethylhexyl) phthalate (DEHP) (117-81-7) Draft p.267 (2001/2003). Available from, as of May 13, 2008: https://esis.jrc.ec.europa.eu/
Two healthy male volunteers (47 and 34 years old) received 30 mg DEHP (> 99% pure) as a single dose or 10 mg/day of DEHP for 4 days ... A urinary elimination half-life of about 12 hours was estimated. ...
European Chemicals Bureau; European Union Risk Assessment Report, Bis(2-ethylhexyl) phthalate (DEHP) (117-81-7) Draft p.262 (2001/2003). Available from, as of May 12, 2008: https://esis.jrc.ec.europa.eu/
For more Biological Half-Life (Complete) data for BIS(2-ETHYLHEXYL) PHTHALATE (9 total), please visit the HSDB record page.
... A single dose of 1000 mg/kg bw MEHP (> 97% purity) in corn oil was administered by gavage to 5-week-old Sprague-Dawley rats, 28-day-old wild-type C57CL/6 mice, or 28-day-old gld mice. The gld mice express a dysfunctional fasL protein, which cannot bind to the fas receptor to initiate apoptosis. ... Following MEHP exposure, apoptosis was found to occur primarily in spermatocytes in both wild-type and gld mice. In wild-type mice, germ cell apoptosis was significantly increased from 6 to 48 hours following MEHP exposure. Apoptotic activity peaked between 12 and 24 hours with 5-fold increases compared to baseline levels. A significant ~2-fold increase in apoptosis compared to baseline levels was observed at 12 and 48 hours following MEHP exposure of gld mice. In both groups of mice, apoptotic activity returned to baseline levels by 96 hours following exposure. Western blot analyses revealed that fas expression significantly increased in wild-type mice (~ 3-fold) at 3 hours following MEHP exposure. There was no significant alteration in fas expression following MEHP exposure in gld mice. Expression of DR4, DR5, and DR6 proteins /which are fas-independent death receptors in the tumor necrosis factor (TNF) superfamily/ occurred in both wild-type and gld mice, but MEHP exposure did not increase expression in either strain. DR5 but not DR4 expression significantly increased in Sprague-Dawley rat testes (~1.5-fold) at 1.5 and 3 hours following MEHP exposure. Procaspase 8 cleavage products, downstream receptor-mediated signals of apoptotic pathways, were detected in testes of wild-type and gld mice, but expression was significantly increased only in gld mice at 6 hours following MEHP exposure. Electrophoretic mobility shift assays demonstrated that DNA binding of NFkappa3, a receptor-mediated downstream signal possibly involved in cell death or survival, was generally reduced in wild-type mice but upregulated in gld mice following MEHP exposure. ... /It was/ concluded that these findings demonstrate that germ-cell related death receptors and downstream signaling products appear to respond to MEHP-induced cell injury ... /MEHP/
NTP/CERHR; Monograph on the Potential Human Reproductive and Developmental Effects of Di(2-ethylhexyl) phthalate (DEHP) p.II-129 (2006) NIH Publication No. 06-4476. Available from, as of May 8, 2008: https://cerhr.niehs.nih.gov/evals/index.html
Sprague-Dawley male rats were treated orally with 250, 500, or 750 mg/kg/d Di(2-ethylhexyl) phthalate (DEHP) for 28 days, while control rats were given corn oil. The levels of cell cycle regulators (pRb, cyclins, CDKs, and p21) and apoptosis-related proteins were analyzed by Western blot analysis. The role of PPAR-gamma (PPAR-gamma), class B scavenger receptor type 1 (SR-B1), and ERK1/2 was further studied to examine the signaling pathway for DEHP-induced apoptosis. Results showed that the levels of pRB, cyclin D, CDK2, cyclin E, and CDK4 were significantly lower in rats given 500 and 750 mg/kg/d DEHP, while levels of p21 were significantly higher in rat testes. Dose-dependent increases in PPAR-gamma and RXRalpha proteins were observed in testes after DEHP exposure, while there was a significant decrease in RXRgamma protein levels. In addition to PPAR-gamma, DEHP also significantly increased SR-B1 mRNA and phosphorylated ERK1/2 protein levels. Furthermore, DEHP treatment induced pro-caspase-3 and cleavage of its substrate protein, poly(ADP-ribose) polymerase (PARP), in a dose-dependent manner. Data suggest that DEHP exposure may induce the expresson of apoptosis-related genes in testes through induction of PPAR-gamma and activation of the ERK1/2 pathway.
PMID:17654247 Ryu JY et al; J Toxicol Environ Health A 70 (15-16): 1296-303 (2007)
Global gene expression profiling combined with an evaluation of Gene Ontology (GO) and pathway mapping tools /was used/ ... for identifying the molecular pathways and processes affected /to examine/ ... the acute effects caused by the non-genotoxic carcinogen and peroxisome proliferator (PP) diethylhexylphthalate (DEHP) in the mouse liver as a model system. Consistent with what is known about the mode of action of DEHP, /the/ GO analysis of transcript profiling data revealed a striking overrepresentation of genes associated with the peroxisomal cellular component, together with genes involved in carboxylic acid and lipid metabolism. Furthermore ... gene expression changes associated with additional biological functions, including complement activation, hemostasis, the endoplasmic reticulum overload response, and circadian rhythm /were revealed/. Together, these data reveal potential new pathways of PP action and shed new light on the mechanisms by which non-genotoxic carcinogens control hepatocyte hypertrophy and proliferation. ...
PMID:15901911 Currie RA et al; Toxicol Sci 86 (2): 453-69 (2005)
... Peroxisome proliferator-activated receptor alpha (PPAR-alpha), the nuclear receptor, is a member of the steroid hormone receptor superfamily and binds to DNA as a heterodimer with the retinoid X receptor (RXR). Peroxisome proliferator response elements (PPREs) have been found in genes for both peroxisomal and microsomal fatty acid-oxidizing enzymes. ... The species differences /responding to peroxisome proliferators, eg DEHP/, particularly with respect to humans compared to rats and mice, can be potentially attributed to ... the level of expression and functional capability of PPAR-alpha, the presence or absence of active PPREs in the promoter region of specific genes, and other aspects of interaction with transcriptional regulatory proteins. ... Marked species differences in the expression of PPAR-alpha mRNA have been demonstrated between rodent and human liver, with the latter expressing 1-10% of the levels found in mouse or rat liver ... PPAR-alpha protein expression /in human livers/ contained less than 10% of the level in mice. ... In most human samples studied, it was found that PPREs are mainly bound by other competing proteins that may block peroxisome proliferator responsiveness. In addition, the low levels of PPAR-alpha protein detected in human liver were lower than those estimated from RNA analysis and this was explained by the finding that a significant fraction of PPAR-alpha mRNA is mis-spliced in human liver. ... The truncated PPAR-alpha mRNA accounted for 25-50% of total PPAR-alpha mRNA in 10 human liver samples, while no truncated PPAR-alpha mRNA was found in livers of rats and mice. The truncated human PPAR-alpha mRNA was expressed in vitro, where it was shown to (a) fail to bind to PPRE, a necessary step for gene activation and (b) interfere with gene activation by expressed full-length human PPAR-alpha, in part due to titration of coactivator CREB-binding protein, an additional element of transcriptional regulation. ... Differential species sensitivity to peroxisome proliferators could depend on gene-specific factors. In the case of peroxisomal acyl-coenzyme A oxidase, the promoter regions containing PPRE responsible for transcriptional activation of the rodent gene are not present in the promoter region of the human gene ... The absence of a significant response of human liver to induction of peroxisome proliferation and hepatocellular proliferation is explained by several aspects of PPAR-alpha-mediated regulation of gene expression. ... Overall, these findings indicate that the increased incidence of liver tumors in mice and rats treated with di(2-ethylhexyl) phthalate results from a mechanism that does not operate in humans.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V77 P118 (2000)
ABOUT THIS PAGE
91
PharmaCompass offers a list of Diethylhexyl Phthalate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Diethylhexyl Phthalate manufacturer or Diethylhexyl Phthalate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Diethylhexyl Phthalate manufacturer or Diethylhexyl Phthalate supplier.
PharmaCompass also assists you with knowing the Diethylhexyl Phthalate API Price utilized in the formulation of products. Diethylhexyl Phthalate API Price is not always fixed or binding as the Diethylhexyl Phthalate Price is obtained through a variety of data sources. The Diethylhexyl Phthalate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Diethylhexyl Phthalate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Diethylhexyl Phthalate, including repackagers and relabelers. The FDA regulates Diethylhexyl Phthalate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Diethylhexyl Phthalate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Diethylhexyl Phthalate supplier is an individual or a company that provides Diethylhexyl Phthalate active pharmaceutical ingredient (API) or Diethylhexyl Phthalate finished formulations upon request. The Diethylhexyl Phthalate suppliers may include Diethylhexyl Phthalate API manufacturers, exporters, distributors and traders.
click here to find a list of Diethylhexyl Phthalate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Diethylhexyl Phthalate DMF (Drug Master File) is a document detailing the whole manufacturing process of Diethylhexyl Phthalate active pharmaceutical ingredient (API) in detail. Different forms of Diethylhexyl Phthalate DMFs exist exist since differing nations have different regulations, such as Diethylhexyl Phthalate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Diethylhexyl Phthalate DMF submitted to regulatory agencies in the US is known as a USDMF. Diethylhexyl Phthalate USDMF includes data on Diethylhexyl Phthalate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Diethylhexyl Phthalate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Diethylhexyl Phthalate suppliers with USDMF on PharmaCompass.
Diethylhexyl Phthalate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Diethylhexyl Phthalate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Diethylhexyl Phthalate GMP manufacturer or Diethylhexyl Phthalate GMP API supplier for your needs.
A Diethylhexyl Phthalate CoA (Certificate of Analysis) is a formal document that attests to Diethylhexyl Phthalate's compliance with Diethylhexyl Phthalate specifications and serves as a tool for batch-level quality control.
Diethylhexyl Phthalate CoA mostly includes findings from lab analyses of a specific batch. For each Diethylhexyl Phthalate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Diethylhexyl Phthalate may be tested according to a variety of international standards, such as European Pharmacopoeia (Diethylhexyl Phthalate EP), Diethylhexyl Phthalate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Diethylhexyl Phthalate USP).
