
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
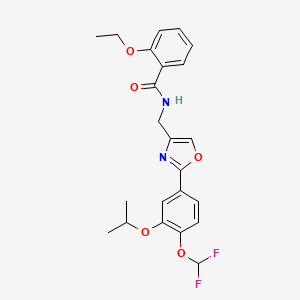

1. Opa-15406
1. Opa-15406
2. 937782-05-3
3. Difamilast [usan]
4. T3u32glj0f
5. N-[[2-[4-(difluoromethoxy)-3-propan-2-yloxyphenyl]-1,3-oxazol-4-yl]methyl]-2-ethoxybenzamide
6. Benzamide, N-((2-(4-(difluoromethoxy)-3-(1-methylethoxy)phenyl)-4-oxazolyl)methyl)-2-ethoxy-
7. Benzamide, N-[[2-[4-(difluoromethoxy)-3-(1-methylethoxy)phenyl]-4-oxazolyl]methyl]-2-ethoxy-
8. Mm36
9. Difamilast [inn]
10. Difamilast [jan]
11. Difamilast (jan/usan)
12. Unii-t3u32glj0f
13. Difamilast [who-dd]
14. Gtpl9776
15. Schembl4275421
16. Chembl3989968
17. Bdbm389126
18. Db14987
19. Usre46792, 352
20. Example 352 [wo2007058338]
21. Hy-109085
22. D11314
23. N-((2-(4-(difluoromethoxy)-3-isopropoxyphenyl)oxazol-4-yl)methyl)-2-ethoxybenzamide
24. N-[2-(4-difluoromethoxy-3-isopropoxyphenyl)oxazol-4-ylmethyl]-2-ethoxybenzamide
25. N-((2-(4-(difluoromethoxy)-3-(propan-2-yloxy)phenyl)-1,3-oxazol-4-yl)methyl)-2-ethoxybenzamide
| Molecular Weight | 446.4 g/mol |
|---|---|
| Molecular Formula | C23H24F2N2O5 |
| XLogP3 | 4.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 10 |
| Exact Mass | 446.16532819 g/mol |
| Monoisotopic Mass | 446.16532819 g/mol |
| Topological Polar Surface Area | 82.8 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 583 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Market Place

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?