
Synopsis
Synopsis
0
CEP/COS
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Canada

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
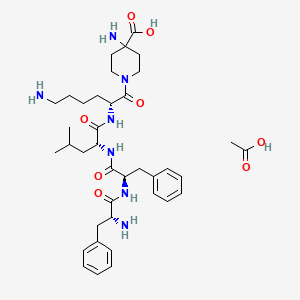

1. 4-piperidinecarboxylic Acid, 4-amino-1-((2r)-6-amino-2-(((2r)-2-(((2r)-2-(((2r)-2-amino-1-oxo-3-phenylpropyl)amino)-1-oxo-3-phenylpropyl)amino)-4-methyl-1-oxopentyl)amino)-1-oxohexyl)-
2. Difelikefalin
3. Korsuva
4. N-((2r)-1-(((2r)-1-(((2r)-6-amino-1-(4-amino-4-carboxy-1-piperidinyl)-1-oxo-2-hexanyl)amino)-4-methyl-1-oxo-2-pentanyl)amino)-1-oxo-3-phenyl-2-propanyl)-d-phenylalaninamide
1. Cr845 Acetate
2. Cr-845 Acetate
3. 1024829-44-4
4. Unii-0p70ar5byb
5. 0p70ar5byb
6. Korsuva
7. 4-amino-1-(d-phenylalanyl-d-phenylalanyl-d-leucyl-d-lysl)-piperidine-4-carboxylic Acid, Acetate Salt
8. Acetic Acid;4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic Acid
9. Difelikefalin (acetate)
10. Chembl5315123
11. Hy-17609c
12. 1024828-77-0 , Difelikefalin
13. Difelikefalin Acetate [who-dd]
14. Ac-38232
15. Ts-10624
16. Difelikefalin Acetate [orange Book]
17. 4-amino-1-(d-phenylalanyl-d-phenylalanyl-d-leucyl-d-lysyl)piperidine-4-carboxylic Acid Acetate
18. N1-(d-phenylalanyl-d-phenylalanyl-d-leucyl-d-lysyl)-4-amino-4-piperidinecarboxylic Acid Acetate (1:)
19. N1-(d-phenylalanyl-d-phenylalanyl-d-leucyl-d-lysyl)-4-amino-4-piperidinecarboxylic Acid Acetate (1:?)
| Molecular Weight | 739.9 g/mol |
|---|---|
| Molecular Formula | C38H57N7O8 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 18 |
| Exact Mass | Da |
| Monoisotopic Mass | Da |
| Topological Polar Surface Area | 260 |
| Heavy Atom Count | 53 |
| Formal Charge | 0 |
| Complexity | 1110 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
