
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Aldizem
2. Cardil
3. Cardizem
4. Crd 401
5. Crd-401
6. Crd401
7. Dilacor
8. Dilacor Xr
9. Dilren
10. Diltiazem Hydrochloride
11. Diltiazem Malate
12. Dilzem
13. Tiazac
1. 42399-41-7
2. D-cis-diltiazem
3. Cardizem
4. Dilt-cd
5. Diltiazemum
6. Anoheal
7. (+)-diltiazem
8. Diltzac
9. Tiamate
10. Cardil
11. Dilren
12. Tiazac
13. Surazem
14. Diltiazem Free Base
15. (+)-cis-diltiazem
16. Diltiazen
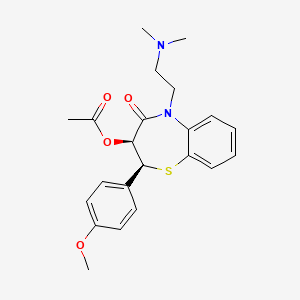
17. (2s,3s)-5-(2-(dimethylamino)ethyl)-2-(4-methoxyphenyl)-4-oxo-2,3,4,5-tetrahydrobenzo[b][1,4]thiazepin-3-yl Acetate
18. Diltiazem (inn)
19. Chembl23
20. Dilcontin
21. Diltiazem Extended Release
22. Dilticard
23. Endrydil
24. Acalix
25. Dilzen
26. Dilta-hexal
27. Ee92bbp03h
28. Incoril Ap
29. Chebi:101278
30. 42399-41-7 (free Base)
31. (2s,3s)-5-[2-(dimethylamino)ethyl]-2-(4-methoxyphenyl)-4-oxo-2,3,4,5-tetrahydro-1,5-benzothiazepin-3-yl Acetate
32. [(2s,3s)-5-(2-dimethylaminoethyl)-2-(4-methoxyphenyl)-4-oxo-2,3-dihydro-1,5-benzothiazepin-3-yl] Acetate
33. Acetic Acid (2s,3s)-5-(2-dimethylamino-ethyl)-2-(4-methoxy-phenyl)-4-oxo-2,3,4,5-tetrahydro-benzo[b][1,4]thiazepin-3-yl Ester
34. Ncgc00024309-05
35. Diltiazem [inn]
36. Dsstox_cid_2940
37. Diltiazem [inn:ban]
38. Dilacor-xr
39. Dsstox_rid_76797
40. Diltiazemum [inn-latin]
41. Dsstox_gsid_22940
42. (+)-cis-5-[2-(dimethylamino)ethyl]-2,3-dihydro-3-hydroxy-2-(p-methoxyphenyl)-1,5-benzothiazepin-4(5h)-one Acetate Ester
43. (2s-cis)-3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-1,5-benzothiazepin-4(5h)-one
44. [(2~{s},3~{s})-5-[2-(dimethylamino)ethyl]-2-(4-methoxyphenyl)-4-oxidanylidene-2,3-dihydro-1,5-benzothiazepin-3-yl] Ethanoate
45. Surazem (tn)
46. Cas-42399-41-7
47. Hsdb 6528
48. Cardizem (hydrochloride)
49. Mk-793 (malate)
50. Einecs 255-796-4
51. Unii-ee92bbp03h
52. Brn 3573079
53. Tetrahydrobenzo
54. Ditiaz
55. Rg 83606 (hydrochloride)
56. Diltiazem Hci
57. 1,5-benzothiazepin-4(5h)-one, 3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-, (2s,3s)-
58. D-(cis)-diltiazem
59. Tocris-0685
60. Diltiazem [mi]
61. Ditiaz [vandf]
62. Diltiazem [hsdb]
63. 103532-26-9
64. Prestwick0_000134
65. Prestwick1_000134
66. Prestwick2_000134
67. Prestwick3_000134
68. Diltiazem [vandf]
69. Diltiazem [who-dd]
70. Lopac0_000327
71. Schembl17776
72. Bspbio_000208
73. Bspbio_001311
74. Lithiumdicyclohexylamide
75. Bidd:gt0548
76. Spbio_002147
77. Bpbio1_000230
78. Gtpl2298
79. Dtxsid9022940
80. Bio1_000371
81. Bio1_000860
82. Bio1_001349
83. Hms1791b13
84. Hms1989b13
85. Hms2089h09
86. Zinc621893
87. [b][1,4]thiazepin-3-yl Acetate
88. (+)-5-(2-(dimethylamino)ethyl)-cis-2,3-dihydro-3-hydroxy-2-(p-methoxyphenyl)-1,5-benzothiazepin-4(5h)-one Acetate (ester)
89. 56209-45-1
90. Acetic Acid 5-(2-dimethylamino-ethyl)-2-(4-methoxy-phenyl)-4-oxo-2,3,4,5-tetrahydro-benzo[b][1,4]thiazepin-3-yl Ester
91. Cis-3-acetoxy-5-(2-(dimethylamino)ethyl)-2,3-dihydro-2-(4-methoxyphenyl)-1,5-benzothiazepin-4(5h)-one
92. Hy-b0632
93. Einecs 260-060-0
94. Methoxyphenyl)-4-oxo-2,3,4,5-
95. Tox21_110898
96. Ac-936
97. Bdbm50004704
98. Akos015897257
99. Tox21_110898_1
100. Ccg-204422
101. Db00343
102. Sdccgsbi-0050315.p002
103. Ncgc00024309-02
104. Ncgc00024309-04
105. Ncgc00024309-06
106. Ncgc00024309-07
107. Ncgc00024309-08
108. Ncgc00024309-09
109. Ncgc00024309-10
110. Ncgc00024309-11
111. Ncgc00024309-17
112. Ncgc00024309-21
113. Ncgc00024309-27
114. (2s,3s)-5-[2-(dimethylamino)ethyl]-2-[4-(methyloxy)phenyl]-4-oxo-2,3,4,5-tetrahydro-1,5-benzothiazepin-3-yl Acetate
115. [(2s,3s)-5-[2-(dimethylamino)ethyl]-2-(4-methoxyphenyl)-4-oxo-2,3-dihydro-1,5-benzothiazepin-3-yl] Acetate
116. Cs-0009567
117. Ethyl 3-amino-1,2,4-triazole-1-carboxylate
118. C06958
119. D07845
120. (2s,3s)-5-(2-(dimethylamino)ethyl)-2-(4-
121. A825887
122. Q422229
123. W-106274
124. Brd-k24023109-001-02-5
125. Brd-k24023109-003-03-9
126. Brd-k24023109-003-11-2
127. Brd-k24023109-003-20-3
128. 8-chloro-1-methyl-6-phenyl-4h-2,3,5,10b-tetraaza-benzo[e]azulene
129. (2s,3s)-5-(2-(dimethylamino)ethyl)-2-(4-methoxyphenyl)-4-oxo-2,3,4,5-tetrahydrobenzo[b][1,4]thiazepin-3-ylacetate
130. 1,5-benzothiazepin-4(5h)-one, 3-(acetyloxy)-5-(u2-(dimethylamino)ethyl)-2,3-dihydro-2-(4-methoxyphenyl)-, (+)-cis-
131. 5-(2-dimethylamino-ethyl)-2-(4-methoxy-phenyl)-4-oxo-2,3,4,5-tetrahydro-benzo[b][1,4]thiazepin-3-yl Ester(diltiazem)acetic Acid
132. Acetic Acid (s)-5-(2-dimethylamino-ethyl)-2-(4-methoxy-phenyl)-4-oxo-2,3,4,5-tetrahydro-benzo[b][1,4]thiazepin-3-yl Ester
133. Acetic Acid 5-(2-dimethylamino-ethyl)-2-(4-methoxy-phenyl)-4-oxo-2,3,4,5-tetrahydro-benzo[b][1,4]thiazepin-3-yl Ester (diltiazem)
134. Acetic Acid 5-(2-dimethylamino-ethyl)-2-(4-methoxy-phenyl)-4-oxo-2,3,4,5-tetrahydro-benzo[b][1,4]thiazepin-3-yl Ester(cis-(+)-diltiazem)
135. Acetic Acid 5-(2-dimethylamino-ethyl)-2-(4-methoxy-phenyl)-4-oxo-2,3,4,5-tetrahydro-benzo[b][1,4]thiazepin-3-yl Ester; Hydrochloride
136. C9f
137. Cis-acetic Acid 5-(2-dimethylamino-ethyl)-2-(4-methoxy-phenyl)-4-oxo-2,3,4,5-tetrahydro-benzo[b][1,4]thiazepin-3-yl Ester
138. Diltiazem;acetic Acid 5-(2-dimethylamino-ethyl)-2-(4-methoxy-phenyl)-4-oxo-2,3,4,5-tetrahydro-benzo[b][1,4]thiazepin-3-yl Ester
| Molecular Weight | 414.5 g/mol |
|---|---|
| Molecular Formula | C22H26N2O4S |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 7 |
| Exact Mass | 414.16132849 g/mol |
| Monoisotopic Mass | 414.16132849 g/mol |
| Topological Polar Surface Area | 84.4 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 565 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 10 | |
|---|---|
| Drug Name | Cardizem |
| PubMed Health | Diltiazem (Intravenous route) |
| Drug Classes | Benzothiazepine, Calcium Channel Blocker, Cardiovascular Agent |
| Drug Label | CARDIZEM (diltiazem hydrochloride) is a calcium ion cellular influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-Benzothiazepin-4(5H)-one, 3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 60mg; 30mg; 90mg; 120mg |
| Market Status | Prescription |
| Company | Valeant Intl |
| 2 of 10 | |
|---|---|
| Drug Name | Cartia xt |
| PubMed Health | Diltiazem (By mouth) |
| Drug Classes | Antianginal, Antihypertensive, Cardiovascular Agent |
| Drug Label | Diltiazem hydrochloride is a calcium ion cellular influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-benzothiazepin-4(5H)one,3-(acetyloxy)-5-[2-(dimethylamino) ethyl]-2,3-dihydro-2-(4-methoxyphen... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 300mg; 180mg; 120mg; 240mg |
| Market Status | Prescription |
| Company | Actavis Labs Fl |
| 3 of 10 | |
|---|---|
| Drug Name | Dilacor xr |
| Drug Label | DILACOR XR (diltiazem hydrochloride, USP) is a calcium ion influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-Benzothiazepin-4(5H)one,3-(acetyloxy)-5-[2-(dimethylamino) ethyl]-2,3-dihydro-2-(4-... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 180mg; 120mg; 240mg |
| Market Status | Prescription |
| Company | Watson Labs |
| 4 of 10 | |
|---|---|
| Drug Name | Dilt-cd |
| Drug Label | Diltiazem hydrochloride is a calcium ion cellular influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-benzothiazepin-4(5H)one,3-(acetyloxy)-5-[2-(dimethylamino) ethyl]-2,3-dihydro-2-(4-methoxyphen... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 300mg; 180mg; 120mg; 240mg |
| Market Status | Prescription |
| Company | Apotex |
| 5 of 10 | |
|---|---|
| Drug Name | Diltzac |
| Drug Label | Diltiazem hydrochloride is a calcium ion cellular influx inhibitor (slow channel blocker). Chemically, diltiazem hydrochloride is 1,5-benzothiazepin-4(5H)-one,3-(acetyloxy)-5[2-(dimethylamino)ethyl]-2,-3-dihydro-2(4-methoxyphenyl)-, monohydrochloride... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 300mg; 180mg; 120mg; 360mg; 240mg |
| Market Status | Prescription |
| Company | Apotex |
| 6 of 10 | |
|---|---|
| Drug Name | Cardizem |
| PubMed Health | Diltiazem (Intravenous route) |
| Drug Classes | Benzothiazepine, Calcium Channel Blocker, Cardiovascular Agent |
| Drug Label | CARDIZEM (diltiazem hydrochloride) is a calcium ion cellular influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-Benzothiazepin-4(5H)-one, 3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 60mg; 30mg; 90mg; 120mg |
| Market Status | Prescription |
| Company | Valeant Intl |
| 7 of 10 | |
|---|---|
| Drug Name | Cartia xt |
| PubMed Health | Diltiazem (By mouth) |
| Drug Classes | Antianginal, Antihypertensive, Cardiovascular Agent |
| Drug Label | Diltiazem hydrochloride is a calcium ion cellular influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-benzothiazepin-4(5H)one,3-(acetyloxy)-5-[2-(dimethylamino) ethyl]-2,3-dihydro-2-(4-methoxyphen... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 300mg; 180mg; 120mg; 240mg |
| Market Status | Prescription |
| Company | Actavis Labs Fl |
| 8 of 10 | |
|---|---|
| Drug Name | Dilacor xr |
| Drug Label | DILACOR XR (diltiazem hydrochloride, USP) is a calcium ion influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-Benzothiazepin-4(5H)one,3-(acetyloxy)-5-[2-(dimethylamino) ethyl]-2,3-dihydro-2-(4-... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 180mg; 120mg; 240mg |
| Market Status | Prescription |
| Company | Watson Labs |
| 9 of 10 | |
|---|---|
| Drug Name | Dilt-cd |
| Drug Label | Diltiazem hydrochloride is a calcium ion cellular influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-benzothiazepin-4(5H)one,3-(acetyloxy)-5-[2-(dimethylamino) ethyl]-2,3-dihydro-2-(4-methoxyphen... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 300mg; 180mg; 120mg; 240mg |
| Market Status | Prescription |
| Company | Apotex |
| 10 of 10 | |
|---|---|
| Drug Name | Diltzac |
| Drug Label | Diltiazem hydrochloride is a calcium ion cellular influx inhibitor (slow channel blocker). Chemically, diltiazem hydrochloride is 1,5-benzothiazepin-4(5H)-one,3-(acetyloxy)-5[2-(dimethylamino)ethyl]-2,-3-dihydro-2(4-methoxyphenyl)-, monohydrochloride... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 300mg; 180mg; 120mg; 360mg; 240mg |
| Market Status | Prescription |
| Company | Apotex |
Diltiazem ... may reduce the incidence of reinfarction in patients with a first non-Q-wave infarction who are not candidates for a beta-adrenergic receptor antagonist.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 860
... Diltiazem ... /has/ been shown to provide symptomatic relief in Raynaud's disease.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 860
Diltiazem ... /is/ indicated, alone or in combination with other agents, for treatment of hypertension. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional 21 st ed. Volume 1. MICROMEDEX Thomson Health Care, Englewood, CO. 2001. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 730
... Parenteral diltiazem /is/ indicated in the treatment of supraventricular tachyarrhythmias. Diltiazem ... produces rapid conversion to sinus rhythm of paroxysmal supraventricular tachycardia (including those associated with accessory bypass tracts, such as Wolff-Parkinson-White [W-P-W] or Lown-Ganong-Levine [L-G-L] syndrome) in patients who do not respond to vagal maneuvers {161} when the atrioventricular (AV) node is required for reentry to sustain tachycardia {125}. Parenteral diltiazem ... also produces temporary control of rapid ventricular rate in atrial flutter or atrial fibrillation. ... /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional 21 st ed. Volume 1. MICROMEDEX Thomson Health Care, Englewood, CO. 2001. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 730
For more Therapeutic Uses (Complete) data for DILTIAZEM (16 total), please visit the HSDB record page.
The toxic effects of sustained-release calcium channel blockers may be delayed more than 12 hr after ingestion. All patients with sustained-release calcium channel blocker overdose should be admitted to the hospital for observation, even if they are asymptomatic. /Calcium channel blockers/
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 533
Withdrawal of calcium channel blocking drugs from severely hypertensive patients, even in the absence of previous angina or myocardial infarction, may precipitate myocardial infarction. Worsening angina & myocardial infarction have been described after the withdrawal of calcium channel blocking agents in patients with normal coronary angiography who are being treated for ischemic chest pain. /Calcium channel blockers/
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 533
Patients with ventricular dysfunction, SA or AV nodal conduction disturbances, and systolic blood pressures below 90 mm Hg should not be treated with ... diltiazem, particularly iv.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 859
The most common adverse cardiovascular effect noted with IV diltiazem is symptomatic or asymptomatic hypotension, which occurred in 3.2 or 4.3%, respectively, of patients receiving the drug in clinical trials. Hypotension or postural hypotension also was noted in approximately 1% or less of patients receiving oral diltiazem. If symptomatic hypotension occurs, appropriate therapy (e.g., placement of the patients in the Trendelenburg's position, plasma volume expansion) should be initiated. Hypotension occurred secondary to the vasodilating action of diltiazem on vascular smooth muscle. Vasodilation or flushing occurred in 1.7% of patients receiving IV diltiazem and in approximately 1% or less of patients receiving oral diltiazem in clinical trials.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1610
For more Drug Warnings (Complete) data for DILTIAZEM (18 total), please visit the HSDB record page.
At /blood/ levels /of diltiazem/ above 6,100 ug/L, most patients die.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 537
**Oral** Indicated for the management of hypertension, to lower blood pressure, alone or in combination with other antihypertensive agents. Indicated for use to improve exercise tolerance in patients with chronic stable angina. Indicated for the management of variant angina (Prinzmetal's angina). **Intravenous** Indicated for the short-term management of atrial fibrillation or atrial flutter for temporary control of rapid ventricular rate. Indicated for the rapid conversion of paroxysmal supraventricular tachycardias (PSVT) to sinus rhythm. This includes AV nodal reentrant tachycardias and reciprocating tachycardias associated with an extranodal accessory pathway such as the WPW syndrome or short PR syndrome. **Off-label** Indicated for off-label uses in anal fissures (as topical formulation), migraine prophylaxis, cramps in lower leg related to rest, pulmonary hypertension, idiopathic dilated cardiomyopathy, and proteinuria associated with diabetic nephropathy.
Diltiazem is an antihypertensive and vasodilating agent that works by relaxing the vascular muscle and reducing blood pressure. This is related to the long-term therapeutic effects, as lowering the blood pressure reduces the risk of fatal and non-fatal cardiovascular events, primarily strokes and myocardial infarctions. Diltiazem inhibits the influx of extracellular calcium ions across the myocardial and vascular smooth muscle cell membranes during depolarization. Diltiazem is classified as a negative inotrope (decreased force) and negative chronotrope (decreased rate). It is also considered a rate-control drug as it reduces heart rate. Diltiazem is exerts hemodynamic actions by reducing blood pressure, systemic vascular resistance, the rate-pressure product, and coronary vascular resistance while increasing coronary blood flow. Diltiazem decreases sinoatrial and atrioventricular conduction in isolated tissues and has a negative inotropic effect in isolated preparations. In supraventricular tachycardia, diltiazem prolongs AV nodal refractories. As the magnitude of blood pressure reduction is related to the degree of hypertension, the antihypertensive effect of diltiazem is most pronounced in individuals with hypertension. In a randomized, double-blind, parallel-group, dose-response study involving patients with essential hypertension, there was a reduction in the diastolic blood pressure by 1.9, 5.4, 6.1, and 8.6 mmHg in the patients receiving diltiazem at doses of 120, 240, 360, and 540 mg, respectively. In patients receiving placebo, there was a reduction in the diastolic blood pressure by 2.6 mmHg.In a randomized, double-blind study involving patients with chronic stable angina, variable doses of diltiazem administered at night all caused an increased exercise tolerance in the after 21 hours, compared to placebo. In the NORDIL study of patients with hypertension, the therapeutic effectiveness of diltiazem in reducing cardiovascular morbidity and mortality was assessed. When using the combined primary endpoint as fatal and non-fatal stroke, myocardial infarction, and other cardiovascular death, fatal and non-fatal stroke was shown to be reduced by 25% in the diltiazem group. Although the clinical significance to this effect remains unclear, it is suggested that diltiazem may exert a protective role against cerebral stroke in hypertensive patients.
Cardiovascular Agents
Agents that affect the rate or intensity of cardiac contraction, blood vessel diameter, or blood volume. (See all compounds classified as Cardiovascular Agents.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Calcium Channel Blockers
A class of drugs that act by selective inhibition of calcium influx through cellular membranes. (See all compounds classified as Calcium Channel Blockers.)
C - Cardiovascular system
C05 - Vasoprotectives
C05A - Agents for treatment of hemorrhoids and anal fissures for topical use
C05AE - Muscle relaxants
C05AE03 - Diltiazem
C - Cardiovascular system
C08 - Calcium channel blockers
C08D - Selective calcium channel blockers with direct cardiac effects
C08DB - Benzothiazepine derivatives
C08DB01 - Diltiazem
Absorption
Diltiazem is readily absorbed from the gastrointestinal tract. Minimum therapeutic plasma diltiazem concentrations appear to be in the range of 50 to 200 ng/mL. Following oral administration of extended formulations of 360 mg diltiazem, the drug in plasma was detectable within 3 to 4 hours and the peak plasma concentrations were reached between 11 and 18 hours post-dose. Diltiazem peak and systemic exposures were not affected by concurrent food intake. Due to hepatic first-pass metabolism, the absolute bioavailability following oral administration is about 40%, with the value ranging from 24 to 74% due to high interindividual variation in the first pass effect. The bioavailability may increase in patients with hepatic impairment.
Route of Elimination
Due to its extensive metabolism, only 2% to 4% of the unchanged drug can be detected in the urine. The major urinary metabolite in healthy volunnteers was N-monodesmethyl diltiazem, followed by deacetyl N,O-didesmethyl diltiazem, deacetyl N-monodesmethyl diltiazem, and deacetyl diltiazem; however, there seems to be large inter-individual variability in the urinary excretion of DTZ and its metabolites.
Volume of Distribution
The apparent volume of distribution of diltiazem was approximately 305 L following a single intravenous injection in healthy male volunteers.
Clearance
Following a single intravenous injection in healthy male volunteers, the systemic clearance of diltiazem was approximately 65 L/h. After constant rate intravenous infusion, the systemic clearance decreased to 48 L/h.
The protein binding of diltiazem is 80-90%, & the volume of distribution is approx 5.3 L/kg. Clearance of diltiazem after oral ingestion follows first-order kinetics, with a half-life of 5-10 hr, independent of the amount ingested. In sustained release preparations, however, the peak absorption time is delayed & the half-life may be very prolonged because of continued GI absorption.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 533
Although the absorption of ... agents /like diltiazem/ is nearly complete after oral admin, their bioavailability is reduced, in some cases markedly, because of first-pass hepatic metab. The effects of these drugs are evident within 30-60 min of an oral dose ... . During repeated oral admin, bioavailability and half-life may incr because of saturation of hepatic metabolism. A major metabolite of diltiazem is desacetyldiltiazem, which has about 1/2 of diltiazem's potency as a vasodilator.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 858
Diltiazem is excreted into human milk.
Briggs, G.G, R.K. Freeman, S.J. Yaffe. A Reference Guide to Fetal and Neonatal Risk. Drugs in Pregnancy and Lactation. 4th ed. Baltimore, MD: Williams & Wilkins 1994., p. 288
The pharmacokinetic changes of diltiazem ... and its main metabolite, deacetyldiltiazem (DAD) were studied after oral admin of diltiazem to normal rabbits and mild and medium folate-induced renal failure rabbits. Diltiazem 10 mg/kg was given to the rabbits ... orally (n=6). Plasma concns of diltiazem and DAD were determined by a high performance liquid chromatography assay. The area under the plasma concn-time curves (AUC) and max plasma concn (Cmax) of diltiazem were significantly increased in mild and medium folate-induced renal failure rabbits. The metabolite ratio of the diltiazem to DAD were significantly decreased in mild and medium folate-induced renal failure rabbits. The volume of distribution (Vd) and total body clearance (CLt) of diltiazem were significantly decreased in mild and medium folate-induced renal failure rabbits. The elimination rate constant (beta) of diltiazem was significantly decreased in folate-induced renal failure rabbits, but that of DAD was significantly increased. These findings suggest that the hepatic metab of diltiazem was inhibited ... .
PMID:11534767 Choi JS, et al; Arch Pharm Res 24(4): 333-337 (2001)
The pharmacokinetics of diltiazem in rabbits after subconjunctival and topical admin was studied. Diltiazem successfully penetrated the aq humor of rabbit eyes. The peak aq concns were 3.8 +/- 0.4 ug/ml after topical application and 15.3 +/- 1.1 ug/ml after subconjunctival injection. The peak aq concn was achieved 1/2 hrs after admin in both cases.
PMID:10744205 Oruc S, et al; Eur J Ophthalmol 10(1): 46-50 (2000)
Diltiazem is subject to extensive first-pass metabolism, which explains its relatively low absolute oral bioavailability. It undergoes N-demethylation primarily mediated by CYP3A4. CYP2D6 is responsible for O-demethylation and esterases mediate deacetylation. There was large inter-individual variability in the circulating plasma levels of metabolites in healthy volunteers. In healthy volunteers, the major circulating metabolites in the plasma are N-monodesmethyl diltilazem, deacetyl diltiazem, and deacetyl N-monodesmethyl diltiazem, which are all pharmacologically active. Deacetyl diltiazem retains about 25-50% of the pharmacological activity to that of the parent compound. Deacetyl diltiazem can be further transformed into deacetyl diltiazem N-oxide or deacetyl O-desmethyl diltiazem. N-monodesmethyl diltilazem can be further metabolized to N,O-didesmethyl diltiazem. Deacetyl N-monodesmethyl diltiazem can be further metabolized to deacetyl N,O-didesmethyl diltiazem, which can be glucuronidated or sulphated. Diltiazem can be O-demethylated by CYP2D6 to form O-desmethyl diltiazem.
A major metabolite of diltiazem is desacetyldiltiazem, which has about 1/2 of diltiazem's potency as a vasodilator.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 858
Diltiazem undergoes extensive metabolism in hepatic and extrahepatic tissues. Deacetyldiltiazem (M1) and N-demethyldiltiazem (MA) are 2 of the main basic metabolites of diltiazem that retain pharmacological activity. This drug impairs its own metab after chronic admin in the adult patient.
PMID:11465404 Fraile LJ, et al; Xenobiotica 31(4): 177-185 (2001)
Diltiazem has known human metabolites that include N-Demethyldiltiazem and O-Demethyldiltiazem.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The plasma elimination half-life is approximately 3.0 to 4.5 hours following single and multiple oral doses. The half-life may slightly increase with dose and the extent of hepatic impairment. The apparent elimination half-life for diltiazem as extended-release tablets after single or multiple dosing is 6 to 9 hours. The plasma elimination half-life is approximately 3.4 hours following administration of a single intravenous injection. The elimination half-lives of pharmacologically active metabolites are longer than that of diltiazem.
Clearance of diltiazem after oral ingestion follows first-order kinetics, with a half-life of 5-10 hr ... .
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 533
Excitation of cardiac muscle involves the activation of a slow calcium inward current that is induced by L-type slow calcium channels, which are voltage-sensitive, ion-selective channels associated with a high activation threshold and slow inactivation profile. L-type calcium channels are the main current responsible for the late phase of the pacemaker potential. Acting as the main Ca2+ source for contraction in smooth and cardiac muscle, activation of L-type calcium channels allows the influx of calcium ions into the muscles upon depolarization and excitation of the channel. It is proposed that this cation influx may also trigger the release of additional calcium ions from intracellular storage sites. Diltiazem is a slow calcium channel blocker that binds to the extracellular site of the alpha-1C subunit of the channel, which is thought to be the S5-6 linker region of the transmembrane domain IV and/or S6 segment of domain III. Diltiazem can get access to this binding site from either the intracellular or extracellular side, but it requires a voltage-induced conformational changes in the membrane. Diltiazem inhibits the influx of extracellular calcium across the myocardial and vascular smooth muscle cell membranes. In isolated human atrial and ventricular myocardium, diltiazem suppressed tension over the range of membrane potentials associated with calcium channel activity but had little effect on the tension-voltage relations at more positive potentials. This effect is thought to be mediated by the voltage-dependent block of the L-type calcium channels and inhibition of calcium ion release from the ER stores, without altering the sodium-calcium coupled transport or calcium sensitivity of myofilaments. Through inhibition of inward calcium current, diltiazem exerts a direct ionotropic and energy sparing effect on the myocardium. Diltiazem fslows atrioventricular nodal conduction, which is due to its ability to impede slow channel function. Reduced intracellular calcium concentrations equate to increased smooth muscle relaxation resulting in arterial vasodilation and therefore, decreased blood pressure. The decrease in intracellular calcium inhibits the contractile processes of the myocardial smooth muscle cells, causing dilation of the coronary and systemic arteries, increased oxygen delivery to the myocardial tissue, decreased total peripheral resistance, decreased systemic blood pressure, and decreased afterload. Through its actions on reducing calcium levels in cardiac and vascular smooth muscles, diltiazem causes a reduction in the contractile processes of the myocardial smooth muscle cells and vasodilation of the coronary and systemic arteries, including epicardial and subendocardial. This subsequently leads to increased oxygen delivery to the myocardial tissue, improved cardiac output due to increased stroke volume, decreased total peripheral resistance, decreased systemic blood pressure and heart rate, and decreased afterload. Diltiazem lowers myocardial oxygen demand through a reduction in heart rate, blood pressure, and cardiac contractility; this leads to a therapeutic effect in improving exercise tolerance in chronic stable angina.
The effects of D-cis- and L-cis-diltiazem on the hydrogen peroxide (H2O2)-induced derangements of mechanical function and energy metab, and accumulation of intracellular Na+ were studied in isolated rat hearts. The intracellular concn of Na+ ([Na+]i) in the myocardium was measured using a nuclear magnetic resonance technique. H2O2 (600 uM) increased the left ventricular end-diastolic pressure, decreased the tissue level of ATP, and increased the release of lactate dehydrogenase from the myocardium. These alterations induced by H2O2 were significantly attenuated by D-cis-diltiazem (15 uM) or L-cis-diltiazem (15 uM). H2O2 (1 mM) produced a marked incr in the myocardial [Na+]i, which was effectively inhibited by ... D-cis-diltiazem (15 uM) or L-cis-diltiazem (15 uM). ... The protective action of D-cis- and L-cis-diltiazem may be due to their ability to inhibit the H2O2-induced incr in [Na+]i, at least in part.
PMID:10422783 Xiao CY, et al; Eur J Pharmacol 374(3): 387-398 (1999)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
35
PharmaCompass offers a list of Diltiazem API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Diltiazem manufacturer or Diltiazem supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Diltiazem manufacturer or Diltiazem supplier.
PharmaCompass also assists you with knowing the Diltiazem API Price utilized in the formulation of products. Diltiazem API Price is not always fixed or binding as the Diltiazem Price is obtained through a variety of data sources. The Diltiazem Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Diltiazem manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Diltiazem, including repackagers and relabelers. The FDA regulates Diltiazem manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Diltiazem API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Diltiazem manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Diltiazem supplier is an individual or a company that provides Diltiazem active pharmaceutical ingredient (API) or Diltiazem finished formulations upon request. The Diltiazem suppliers may include Diltiazem API manufacturers, exporters, distributors and traders.
click here to find a list of Diltiazem suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Diltiazem DMF (Drug Master File) is a document detailing the whole manufacturing process of Diltiazem active pharmaceutical ingredient (API) in detail. Different forms of Diltiazem DMFs exist exist since differing nations have different regulations, such as Diltiazem USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Diltiazem DMF submitted to regulatory agencies in the US is known as a USDMF. Diltiazem USDMF includes data on Diltiazem's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Diltiazem USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Diltiazem suppliers with USDMF on PharmaCompass.
Diltiazem Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Diltiazem GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Diltiazem GMP manufacturer or Diltiazem GMP API supplier for your needs.
A Diltiazem CoA (Certificate of Analysis) is a formal document that attests to Diltiazem's compliance with Diltiazem specifications and serves as a tool for batch-level quality control.
Diltiazem CoA mostly includes findings from lab analyses of a specific batch. For each Diltiazem CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Diltiazem may be tested according to a variety of international standards, such as European Pharmacopoeia (Diltiazem EP), Diltiazem JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Diltiazem USP).
