
Synopsis
Synopsis
0
VMF
0
Australia

0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
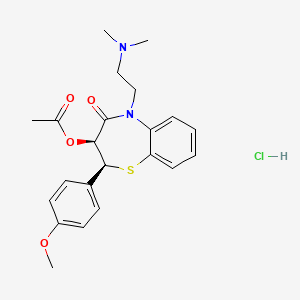

1. Aldizem
2. Cardil
3. Cardizem
4. Crd 401
5. Crd-401
6. Crd401
7. Dilacor
8. Dilacor Xr
9. Dilren
10. Diltiazem
11. Diltiazem Malate
12. Dilzem
13. Tiazac
1. 33286-22-5
2. Diltiazem Hcl
3. Cardizem
4. Dilzem
5. Dilzene
6. Masdil
7. (+)-cis-diltiazem Hydrochloride
8. Tiazac
9. Crd-401
10. Altiazem
11. Deltazen
12. Diladel
13. Dilpral
14. Dilrene
15. Tildiem
16. Adizem
17. Herbesser
18. Dilacor
19. Lacerol
20. Zilden
21. Mono-tildiem
22. Bi-tildiem
23. Cardizem Cd
24. Cardizem La
25. Cardil
26. Dilren
27. Dilthiazem Hydrochloride
28. Diltiazem (hydrochloride)
29. Diltzac
30. Cartia
31. Rg 83606
32. (2s,3s)-5-(2-(dimethylamino)ethyl)-2-(4-methoxyphenyl)-4-oxo-2,3,4,5-tetrahydrobenzo[b][1,4]thiazepin-3-yl Acetate Hydrochloride
33. Taztia
34. Diltiazem Hcl (tiazac)
35. 103532-26-9
36. Mls000028432
37. Olh94387te
38. Rg 83606 Hcl
39. Chebi:645509
40. Anoheal
41. Nsc-759576
42. (+)-5-(2-(dimethylamino)ethyl)-cis-2,3-dihydro-3-hydroxy-2-(p-methoxyphenyl)-1,5-benzothiazepin-4(5h)-one Acetate (ester) Monohydrochloride
43. (2s,3s)-5-[2-(dimethylamino)ethyl]-2-(4-methoxyphenyl)-4-oxo-2,3,4,5-tetrahydro-1,5-benzothiazepin-3-yl Acetate Hydrochloride
44. [(2s,3s)-5-[2-(dimethylamino)ethyl]-2-(4-methoxyphenyl)-4-oxo-2,3-dihydro-1,5-benzothiazepin-3-yl] Acetate;hydrochloride
45. 38411-61-9
46. Dilzicardin
47. Anginyl
48. Angitil
49. Angizem
50. Britiazim
51. Calcicard
52. Calnurs
53. Cardiazem
54. Cirilen
55. Citizen
56. Clarute
57. Corazet
58. Dilatam
59. Dilatame
60. Dilcard
61. Dilgard
62. Dilicardin
63. Diltahexal
64. Diltelan
65. Diltiasyn
66. Diltikor
67. Diltime
68. Dinisor
69. Dodexen
70. Entrydil
71. Farmabes
72. Gadoserin
73. Helsibon
74. Incoril
75. Kaltiazen
76. Levozem
77. Longazem
78. Lytelsen
79. Metazem
80. Miocardie
81. Oxycardil
82. Pazeadin
83. Pentilzeno
84. Poltiazem
85. Presoken
86. Rg-83606
87. Smr000058375
88. Tilazem
89. Trumsal
90. Ziruvate
91. Bruzem
92. Carzem
93. Diatal
94. Dilfar
95. Dilsal
96. Diltam
97. Diltan
98. Doclis
99. Dyalac
100. Etizen
101. Etyzen
102. Herben
103. Kardil
104. Myonil
105. Slozen
106. Tiadil
107. Tiaves
108. Ubicor
109. Zildem
110. Carex
111. Coras
112. Dazil
113. Dilem
114. Dilso
115. Hesor
116. Tazem
117. Apo-diltiazem
118. Iski
119. Altiazem Retard
120. Cardizem Retard
121. Diltiazem Merck
122. Diltiazem Stada
123. Diltiazem Verla
124. Diltiazem-mepha
125. 33286-22-5 (hcl)
126. Cardil Retard
127. Dil-sonaramia
128. Diltiazem-isis
129. Dilzem Retard
130. Dinisor Retard
131. Myonil Retard
132. Novo-diltazem
133. Syn-diltiazem
134. Tildiem Retard
135. Diltiazem Basics
136. Diltiazem-cophar
137. Mfcd00069252
138. Uni Masdil
139. Wl Diltiazem
140. Altiazem Rr
141. Cardizem Sr
142. Diltiazem Awd
143. Diltiazem Gnr
144. Diltiazem Msd
145. Diltiazem-gry
146. Cirilen Ap
147. Diltiazem Henning
148. Diltiazem Upsa
149. Tildiem Cr
150. Tildiem La
151. Adizem-cd
152. Cartia Xt
153. Diltan Sr
154. Diltiazem Eu Rho
155. Dilzem Rr
156. Taztia Xt
157. Viazem Sr
158. Viazem Xl
159. Herbesser 60
160. (2s-cis)-3-acetoxy-5-(2-(dimethylamino)ethyl)-2,3-dihydro-2-(4-methoxyphenyl)-1,5-benzothiazepin-4(5h)-one Monohydrochloride
161. [(2s,3s)-5-[2-(dimethylamino)ethyl]-2-(4-methoxyphenyl)-4-oxo-2,3-dihydro-1,5-benzothiazepin-3-yl] Acetate;hydron;chloride
162. 1,5-benzothiazepin-4(5h)-one, 3-(acetyloxy)-5-(2-(dimethylamino)ethyl)-2,3-dihydro-2-(4-methoxyphenyl)-, Monohydrochloride, (+)-cis-
163. Herbesser 90 Sr
164. Tilazem 90
165. Diltiazem Chloridrate
166. Dilatam 120
167. Tilazem As 60
168. Tilazem As 90
169. Dilzereal 90 Retard
170. Herbesser 180 Sr
171. Dolizem
172. Iski-90 Sr
173. Diltia Xt
174. Dilt-xr
175. Dodexen A.p.
176. Presokin A. P.
177. Mk-793
178. Crd 401
179. Tiazac Extended Release
180. Sr-01000003042
181. Einecs 251-443-3
182. Dilacor Xr Extended Release Capsules
183. Unii-olh94387te
184. Milptin
185. Carzen
186. Slozem
187. Crd401
188. Dilthiazem Hcl
189. Dov Diltiazem
190. Uni-masdil
191. Cardizem Xl
192. Tiazac Xc
193. Cardizem (tn)
194. Prestwick_176
195. Acetate Hydrochloride
196. Einecs 253-918-0
197. Dilacor Xr (tn)
198. Cartia Xt (tn)
199. Opera_id_79
200. Diltiazem Hydrochloride [usan:usp:jan]
201. Dilt-cd (tn)
202. (+)-5-(2-(dimethylamino)ethyl)-cis-2,3-dihydro-3-hydroxy-2-(p-methoxyphenyl)-1,5-benzothiazepin-4(5h)-one Acetate Ester Monohydrochloride
203. 2-[(2s,3s)-3-(acetyloxy)-2-(4-methoxyphenyl)-4-oxo-3,4-dihydro-1,5-benzothiazepin-5(2h)-yl]-n,n-dimethylethanaminium Chloride
204. Chembl1697
205. Diltiazem Hydrochloride,(s)
206. Schembl15457
207. Mls001148257
208. Mls002222179
209. Diltiazem Hcl [vandf]
210. (+)-cis-diltiazem Hydrochlorid
211. Diltiazem For System Suitability
212. Dtxsid8040147
213. Amy8835
214. Slv-324
215. Ven-307
216. Hms1568k10
217. (2s-trans)-diltiazem Hydrochloride
218. Act02682
219. Bcp13814
220. Diltiazem Hydrochloride [mi]
221. Tox21_500327
222. Bnp-32762
223. Diltiazem Hydrochloride (jp17/usp)
224. Diltiazem Hydrochloride [jan]
225. S1865
226. 3,3,3-phosphinylidynetrispropionamide
227. Diltiazem Hydrochloride [usan]
228. Akos015961992
229. Ccg-220134
230. Ccg-221631
231. Diltiazem Hydrochloride [mart.]
232. Diltiazem Hydrochloride [vandf]
233. Ks-5089
234. Lp00327
235. Nc00558
236. Nsc 759576
237. Diltiazem Hydrochloride [usp-rs]
238. Diltiazem Hydrochloride [who-dd]
239. Tetrahydrobenzo[b][1,4]thiazepin-3-yl
240. Ncgc00093768-01
241. Ncgc00261012-01
242. (2s,3s)-(+)-3-acetoxy-2,3-dihydro-5-[2-(dimethylamino)ethyl]-2-(4-methoxyphenyl)-1,5-benzothiazepin-4(5h)-one Hydrochloride
243. 1,5-benzothiazepin-4(5h)-one, 2,3-dihydro-3-(acetyloxy)-5-(2-(dimethylamino)ethyl)-2-(4-methoxyphenyl)-, Monohydrochloride, Cis-(+)-
244. Ac-15189
245. Ac-32469
246. As-13703
247. Bd166408
248. Cis-(1)-3-acetoxy-5-(2-(dimethylamino)ethyl)-2,3-dihydro-2-(4-methoxyphenyl)-1,5-benzothiazepin-4(5h)-one Monohydrochloride
249. Hy-14656
250. (+)-cis-diltiazem Hydrochloride, >/=98%
251. (2s,3s)-5-(2-(dimethylamino)ethyl)-2
252. D3662
253. Diltiazem Hydrochloride [ep Impurity]
254. Diltiazem Hydrochloride [orange Book]
255. Eu-0100327
256. Sw196487-3
257. Diltiazem Hydrochloride [ep Monograph]
258. Bim-0050315.0001
259. D 2521
260. D00616
261. Diltiazem Hydrochloride [usp Monograph]
262. Diltiazem, Hydrochloride - Cas 33286-22-5
263. F20448
264. M01693
265. (+)-cis-diltiazem Hydrochloride, >=99% (hplc)
266. 286d225
267. Sr-01000075327
268. J-019130
269. Sr-01000003042-2
270. Sr-01000003042-4
271. Sr-01000075327-1
272. Q27105183
273. Diltiazem Hydrochloride 1.0 Mg/ml In Acetonitrile (as Free Base)
274. Diltiazem Hydrochloride, European Pharmacopoeia (ep) Reference Standard
275. Diltiazem Hydrochloride, United States Pharmacopeia (usp) Reference Standard
276. Diltiazem For System Suitability, European Pharmacopoeia (ep) Reference Standard
277. (2s,3s)-5-(2-(dimethylamino)ethyl)-2-(4-methoxyphenyl)-4-oxo-2,3,4,5-tetrahydrobenzo[b][1,4]thiazepin-3-yl Acetate Hcl
278. (2s,3s)-5-(2-(dimethylamino)ethyl)-2-(4-methoxyphenyl)-4-oxo-2,3,4,5-tetrahydrobenzo[b][1,4]thiazepin-3-ylacetatehydrochloride
279. (2s-cis)-3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-1,5-benzothiazepin-4(5h)-one Hydrochloride
280. 1,5-benzothiazepin-4(5h)-one, 3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-, Hydrochloride (1:1), (2s,3s)-
281. Acetic Acid (2s,3s)-5-(2-dimethylamino-ethyl)-2-(4-methoxy-phenyl)-4-oxo-2,3,4,5-tetrahydro-benzo[b][1,4]thiazepin-3-yl Ester; Hydrochloride
282. Cis-(+)-3-acetoxy-2,3-dihydro-5-[2-(dimethylamino)ethyl]-2-(4-methoxyphenyl)-1,5-benzothiazepine-4(5h)-one Hydrochloride
283. Diltiazem Hydrochloride Solution, 1.0 Mg/ml In Acetonitrile (as Free Base), Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 451.0 g/mol |
|---|---|
| Molecular Formula | C22H27ClN2O4S |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 7 |
| Exact Mass | 450.1380062 g/mol |
| Monoisotopic Mass | 450.1380062 g/mol |
| Topological Polar Surface Area | 84.4 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 565 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 16 | |
|---|---|
| Drug Name | Cardizem |
| PubMed Health | Diltiazem |
| Drug Classes | Antianginal, Antihypertensive, Benzothiazepine, Calcium Channel Blocker, Cardiovascular Agent |
| Drug Label | CARDIZEM (diltiazem hydrochloride) is a calcium ion cellular influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-Benzothiazepin-4(5H)-one, 3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 60mg; 30mg; 90mg; 120mg |
| Market Status | Prescription |
| Company | Valeant Intl |
| 2 of 16 | |
|---|---|
| Drug Name | Cardizem cd |
| Drug Label | CARDIZEM (diltiazem hydrochloride) is a calcium ion influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-Benzothiazepin-4(5H)-one, 3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2, 3-dihydro-2-(4-metho... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 300mg; 180mg; 120mg; 360mg; 240mg |
| Market Status | Prescription |
| Company | Valeant Intl |
| 3 of 16 | |
|---|---|
| Drug Name | Cardizem la |
| PubMed Health | Diltiazem |
| Drug Classes | Antianginal, Antihypertensive, Benzothiazepine, Calcium Channel Blocker, Cardiovascular Agent |
| Drug Label | CARDIZEM LA (diltiazem hydrochloride) is a calcium ion cellular influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-benzothiazepin-4(5H)-one, 3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 300mg; 180mg; 420mg; 120mg; 360mg; 240mg |
| Market Status | Prescription |
| Company | Valeant Intl |
| 4 of 16 | |
|---|---|
| Drug Name | Cartia xt |
| PubMed Health | Diltiazem (Intravenous route) |
| Drug Classes | Benzothiazepine, Calcium Channel Blocker, Cardiovascular Agent |
| Drug Label | Diltiazem hydrochloride is a calcium ion cellular influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-benzothiazepin-4(5H)one,3-(acetyloxy)-5-[2-(dimethylamino) ethyl]-2,3-dihydro-2-(4-methoxyphen... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 300mg; 180mg; 120mg; 240mg |
| Market Status | Prescription |
| Company | Actavis Labs Fl |
| 5 of 16 | |
|---|---|
| Drug Name | Dilacor xr |
| Drug Label | DILACOR XR (diltiazem hydrochloride, USP) is a calcium ion influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-Benzothiazepin-4(5H)one,3-(acetyloxy)-5-[2-(dimethylamino) ethyl]-2,3-dihydro-2-(4-... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 180mg; 120mg; 240mg |
| Market Status | Prescription |
| Company | Watson Labs |
| 6 of 16 | |
|---|---|
| Drug Name | Diltiazem hydrochloride |
| Drug Label | Diltiazem is a calcium ion influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-Benzothiazepin-4(5H)one,3-(acetyloxy)-5-[2-(dimethylamino) ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-, monohydrochloride... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Tablet, extended release; Injectable; Tablet; Capsule, extended release |
| Route | injection; Injection; Oral |
| Strength | 300mg; 50mg/10ml; 180mg; 100mg/vial; 5mg/ml; 30mg; 60mg; 90mg; 125mg/25ml; 420mg; 120mg; 10mg/ml; 360mg; 240mg |
| Market Status | Prescription |
| Company | Intl Medication; Bedford; Valeant Intl; Hospira; Hikma Maple; Actavis Labs Fl; Teva; Apotex; Hikma Farmaceutica; Teva Pharms Usa; Sandoz; Sun Pharma Global; Par Pharm; Mylan; Actavis; Akorn |
| 7 of 16 | |
|---|---|
| Drug Name | Taztia xt |
| Drug Label | TAZTIA XT brand ofDILTIAZEM HYDROCHLORIDE EXTENDED-RELEASECAPSULES USP(ONCE-A-DAY DOSAGE)Rx Only... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 180mg; 120mg; 360mg; 300mg; 240mg |
| Market Status | Prescription |
| Company | Actavis Labs Fl |
| 8 of 16 | |
|---|---|
| Drug Name | Tiazac |
| Drug Label | Tiazac (diltiazem hydrochloride) is a calcium ion cellular influx inhibitor (slow channel blocker). Chemically, diltiazem hydrochloride is 1,5-Benzothiazepin-4(5H)-one, 3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2, 3-dihydro-2-(4-methoxyphenyl)-, mon... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 180mg; 420mg; 120mg; 360mg; 300mg; 240mg |
| Market Status | Prescription |
| Company | Valeant Intl |
| 9 of 16 | |
|---|---|
| Drug Name | Cardizem |
| PubMed Health | Diltiazem |
| Drug Classes | Antianginal, Antihypertensive, Benzothiazepine, Calcium Channel Blocker, Cardiovascular Agent |
| Drug Label | CARDIZEM (diltiazem hydrochloride) is a calcium ion cellular influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-Benzothiazepin-4(5H)-one, 3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 60mg; 30mg; 90mg; 120mg |
| Market Status | Prescription |
| Company | Valeant Intl |
| 10 of 16 | |
|---|---|
| Drug Name | Cardizem cd |
| Drug Label | CARDIZEM (diltiazem hydrochloride) is a calcium ion influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-Benzothiazepin-4(5H)-one, 3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2, 3-dihydro-2-(4-metho... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 300mg; 180mg; 120mg; 360mg; 240mg |
| Market Status | Prescription |
| Company | Valeant Intl |
| 11 of 16 | |
|---|---|
| Drug Name | Cardizem la |
| PubMed Health | Diltiazem |
| Drug Classes | Antianginal, Antihypertensive, Benzothiazepine, Calcium Channel Blocker, Cardiovascular Agent |
| Drug Label | CARDIZEM LA (diltiazem hydrochloride) is a calcium ion cellular influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-benzothiazepin-4(5H)-one, 3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 300mg; 180mg; 420mg; 120mg; 360mg; 240mg |
| Market Status | Prescription |
| Company | Valeant Intl |
| 12 of 16 | |
|---|---|
| Drug Name | Cartia xt |
| PubMed Health | Diltiazem (Intravenous route) |
| Drug Classes | Benzothiazepine, Calcium Channel Blocker, Cardiovascular Agent |
| Drug Label | Diltiazem hydrochloride is a calcium ion cellular influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-benzothiazepin-4(5H)one,3-(acetyloxy)-5-[2-(dimethylamino) ethyl]-2,3-dihydro-2-(4-methoxyphen... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 300mg; 180mg; 120mg; 240mg |
| Market Status | Prescription |
| Company | Actavis Labs Fl |
| 13 of 16 | |
|---|---|
| Drug Name | Dilacor xr |
| Drug Label | DILACOR XR (diltiazem hydrochloride, USP) is a calcium ion influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-Benzothiazepin-4(5H)one,3-(acetyloxy)-5-[2-(dimethylamino) ethyl]-2,3-dihydro-2-(4-... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 180mg; 120mg; 240mg |
| Market Status | Prescription |
| Company | Watson Labs |
| 14 of 16 | |
|---|---|
| Drug Name | Diltiazem hydrochloride |
| Drug Label | Diltiazem is a calcium ion influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-Benzothiazepin-4(5H)one,3-(acetyloxy)-5-[2-(dimethylamino) ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-, monohydrochloride... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Tablet, extended release; Injectable; Tablet; Capsule, extended release |
| Route | injection; Injection; Oral |
| Strength | 300mg; 50mg/10ml; 180mg; 100mg/vial; 5mg/ml; 30mg; 60mg; 90mg; 125mg/25ml; 420mg; 120mg; 10mg/ml; 360mg; 240mg |
| Market Status | Prescription |
| Company | Intl Medication; Bedford; Valeant Intl; Hospira; Hikma Maple; Actavis Labs Fl; Teva; Apotex; Hikma Farmaceutica; Teva Pharms Usa; Sandoz; Sun Pharma Global; Par Pharm; Mylan; Actavis; Akorn |
| 15 of 16 | |
|---|---|
| Drug Name | Taztia xt |
| Drug Label | TAZTIA XT brand ofDILTIAZEM HYDROCHLORIDE EXTENDED-RELEASECAPSULES USP(ONCE-A-DAY DOSAGE)Rx Only... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 180mg; 120mg; 360mg; 300mg; 240mg |
| Market Status | Prescription |
| Company | Actavis Labs Fl |
| 16 of 16 | |
|---|---|
| Drug Name | Tiazac |
| Drug Label | Tiazac (diltiazem hydrochloride) is a calcium ion cellular influx inhibitor (slow channel blocker). Chemically, diltiazem hydrochloride is 1,5-Benzothiazepin-4(5H)-one, 3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2, 3-dihydro-2-(4-methoxyphenyl)-, mon... |
| Active Ingredient | Diltiazem hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 180mg; 420mg; 120mg; 360mg; 300mg; 240mg |
| Market Status | Prescription |
| Company | Valeant Intl |
Treatment of chronic anal fissure
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Calcium Channel Blockers
A class of drugs that act by selective inhibition of calcium influx through cellular membranes. (See all compounds classified as Calcium Channel Blockers.)
Cardiovascular Agents
Agents that affect the rate or intensity of cardiac contraction, blood vessel diameter, or blood volume. (See all compounds classified as Cardiovascular Agents.)
GDUFA
DMF Review : Complete
Rev. Date : 2017-02-01
Pay. Date : 2016-12-29
DMF Number : 5717
Submission : 1985-02-06
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2021-03-18
Pay. Date : 2021-03-15
DMF Number : 6761
Submission : 1987-01-05
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2013-01-18
Pay. Date : 2012-12-12
DMF Number : 7600
Submission : 1988-07-18
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 6686
Submission : 1986-11-14
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 7887
Submission : 1989-02-07
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 3059
Submission : 1977-11-11
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 7540
Submission : 1988-06-16
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2012-11-29
Pay. Date : 2012-11-08
DMF Number : 7239
Submission : 1987-11-30
Status : Active
Type : II

USDMF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 7240
Submission : 1987-10-26
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Certificate Number : R1-CEP 1998-115 - Rev 05
Status : Valid
Issue Date : 2018-06-29
Type : Chemical
Substance Number : 1004
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 1999-156 - Rev 10
Status : Valid
Issue Date : 2020-02-24
Type : Chemical
Substance Number : 1004

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 1998-036 - Rev 08
Status : Valid
Issue Date : 2024-05-24
Type : Chemical
Substance Number : 1004

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R0-CEP 2020-181 - Rev 00
Status : Valid
Issue Date : 2023-01-11
Type : Chemical
Substance Number : 1004

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2001-177 - Rev 05
Status : Withdrawn by Holder
Issue Date : 2015-10-19
Type : Chemical
Substance Number : 1004

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Diltiazem hydrochloride, Process B
Certificate Number : R1-CEP 2015-260 - Rev 01
Status : Valid
Issue Date : 2022-01-07
Type : Chemical
Substance Number : 1004

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 1997-121 - Rev 14
Status : Valid
Issue Date : 2022-01-07
Type : Chemical
Substance Number : 1004

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 1997-077 - Rev 14
Status : Valid
Issue Date : 2021-12-13
Type : Chemical
Substance Number : 1004


Certificate Number : R1-CEP 2002-245 - Rev 04
Status : Withdrawn by Holder
Issue Date : 2015-10-08
Type : Chemical
Substance Number : 1004


Certificate Number : R1-CEP 1998-135 - Rev 02
Status : Withdrawn by Holder
Issue Date : 2009-06-29
Type : Chemical
Substance Number : 1004

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] 
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Reply
18 Jul 2023
Reply
28 Feb 2023
Reply
13 Feb 2023
Reply
17 Feb 2022
Reply
27 Aug 2021
Reply
07 Aug 2021
Reply
11 Jan 2021
Reply
25 Nov 2019
Reply
16 Oct 2019
Reply
20 Sep 2019
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?