
Synopsis
Synopsis
0
JDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Barosmin
2. Buchu Resin
3. Daflon
4. Resin, Buchu
5. Venosmine
1. 520-27-4
2. Barosmin
3. Diosimin
4. Venosmine
5. Diosmil
6. Diosmine
7. Flebosten
8. Daflon
9. Tovene
10. Ven-detrex
11. Diovenor
12. Buchu Resin
13. Litosmil
14. Veno-v
15. Flebosmil
16. Diosminum
17. Rioven
18. Diosmetin 7-o-rutinoside
19. Diosven
20. Flebaven
21. Flebavena
22. Hemerven
23. Insuven
24. Varinon
25. Dioven
26. Diosmetin-7-o-rutinoside
27. Diosmin [inn]
28. Diosmetin 7-rutinoside
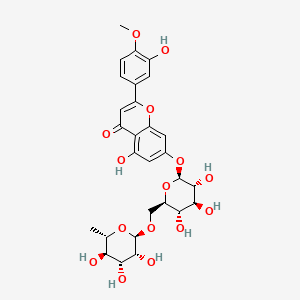
29. 5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-[(2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-[[(2r,3r,4r,5r,6s)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one
30. 7qm776wj5n
31. Chebi:4631
32. 3',5,7-trihydroxy-4'-methoxyflavone 7-rutinoside
33. 3',5,7-trihydroxy-4'-methoxyflavone-7-rutinoside
34. Diosmin (inn)
35. Nsc-758417
36. 5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-(((2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-((((2r,3r,4r,5r,6s)-3,4,5-trihydroxy-6-methyltetrahydro-2h-pyran-2-yl)oxy)methyl)tetrahydro-2h-pyran-2-yl)oxy)-4h-chromen-4-one
37. Diosmin, Analytical Standard
38. Dsstox_cid_25892
39. Dsstox_rid_81206
40. Dsstox_gsid_45892
41. 3',5,7-trihydroxy-4'-methoxyflavone-7-(6-o-(-deoxy-alpha-l-mannopyraonsyl)-beta-d-glucopyranoside
42. 5-hydroxy-2-(3-hydroxy-4-methoxy-phenyl)-7-[(2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-[[(2r,3r,4r,5r,6s)-3,4,5-trihydroxy-6-methyl-tetrahydropyran-2-yl]oxymethyl]tetrahydropyran-2-yl]oxy-chromen-4-one
43. 3',5,7-trihydroxy-4'-methoxyflavone 7-rhamnoglucoside
44. Cas-520-27-4
45. Diosmin [inn:ban]
46. Diosmin [inn-spanish]
47. Diosmine [inn-french]
48. Diosminum [inn-latin]
49. Sr-01000799147
50. Unii-7qm776wj5n
51. Ccris 7915
52. Ncgc00095022-01
53. 4h-1-benzopyran-4-one, 7-((6-o-(6-deoxy-.alpha.-l-mannopyranosyl)-.beta.-d-glucopyranosyl)oxy)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-
54. 4h-1-benzopyran-4-one, 7-[[6-o-(6-deoxy-.alpha.-l-mannopyranosyl)-.beta.-d-glucopyranosyl]oxy]-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-
55. Einecs 208-289-7
56. Daflon (tn)
57. Mfcd00009772
58. Diosmin (8)
59. Se 4601
60. Diosmine [inci]
61. Diosmin [dsc]
62. Diosmin [mi]
63. Diosmin [mart.]
64. Diosmin [usp-rs]
65. Diosmin [who-dd]
66. Mls001304032
67. Schembl120870
68. Diosmin [ep Monograph]
69. Chembl231884
70. Dtxsid4045892
71. 3',5-dihydroxy-4'-methoxy-4-oxo-4h-chromen-7-ylrutosid
72. Bdbm153267
73. Hms2233p16
74. Hms3713l08
75. Act05288
76. Zinc4098512
77. Tox21_111392
78. 3',5-dihydroxy-4'-methoxy-4-oxo-4h-chromen-7-ylrutosid [iupac]
79. S2292
80. Akos015969767
81. Tox21_111392_1
82. Bcp9000612
83. Ccg-208570
84. Db08995
85. Nsc 758417
86. Smp1_000183
87. Ncgc00344564-01
88. 4h-1-benzopyran-4-one, 7-((6-o-(6-deoxy-alpha-l-mannopyranosyl)-beta-d-glucopyranosyl)oxy)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-
89. 4h-1-benzopyran-4-one,7-[[6-o-(6-deoxy-a-l-mannopyranosyl)-b-d-glucopyranosyl]oxy]-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-
90. 5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4-oxo-4h-1-benzopyran-7-yl 6-o-(6-deoxy-alpha-l-mannopyranosyl)-beta-d-glucopyranoside
91. 5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-((6-o-alpha-l-rhamnopyranosyl-beta-d-glycopyranosyl)oxy)-4-chromenon
92. As-13224
93. Bp-12422
94. Smr000718616
95. Bcp0726000067
96. C10039
97. D07858
98. 520d274
99. 3',5,7-trihydroxy-4'-methoxy Flavone-7-rutinoside
100. Q2607865
101. Sr-01000799147-4
102. Sr-01000799147-5
103. Sr-01000799147-6
104. Diosmin, European Pharmacopoeia (ep) Reference Standard
105. Diosmin, United States Pharmacopeia (usp) Reference Standard
106. Diosmin For System Suitability, European Pharmacopoeia (ep) Reference Standard
107. 5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-((2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(((2r,3r,4r,5r,6s)-3,4,5-trihydroxy-6-methyltetrahydro-2h-pyran-2-yloxy)methyl)tetrahydro-2h-pyran-2-yloxy)-4h-chromen-4-one
108. 5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-(o6-alpha-l-rhamnopyranosyl-beta-d-glucopyranosyloxy)chromen-4-one
109. 5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-[(2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-[[(2s,3s,4s,5s,6r)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one
110. 7-((6-o-(6-deoxy-ga-l-mannopyranosyl)-.beta.-d-glucopyranosyl)oxy)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4h-1-benzopyran-4-one
111. 7-((6-o-(6-deoxy-ga-l-mannopyranosyl)-beta-d-glucopyranosyl)oxy)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4h-1-benzopyran-4-one
| Molecular Weight | 608.5 g/mol |
|---|---|
| Molecular Formula | C28H32O15 |
| XLogP3 | -0.8 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 7 |
| Exact Mass | 608.17412031 g/mol |
| Monoisotopic Mass | 608.17412031 g/mol |
| Topological Polar Surface Area | 234 Ų |
| Heavy Atom Count | 43 |
| Formal Charge | 0 |
| Complexity | 995 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 10 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Diosmin is used over-the-counter alone or with ingredients such as [hesperidin] and [diosmetin] to support vein and capillary function.
Diosmin is a venoactive drug supporting circulatory health through various actions on blood vessels; it supports lymphatic drainage and improves microcirculation while increasing venous tone and elasticity. For these reasons, diosmin is frequently taken by individuals with chronic venous disease to support vascular health and has been demonstrated to improve quality of life. In addition to the above effects, diosmin exerts antioxidant activity and scavenges oxygen free radicals, reducing levels of oxidative stress normally detected through biomarkers such as prostaglandins isoprostane precursors. In one clinical study, mean content of TNF alpha, VEGF-C, VEGF-A IL-6, in addition to FGF2 were decreased by after the therapy with diosmin; findings were statistically significant. Additionally, a decrease in edema and mean leg circumference of patients taking diosmin for three months was observed in a clinical study. Diosmin has been demonstrated to enhance the metabolism of glucose in diabetic disorders.
C - Cardiovascular system
C05 - Vasoprotectives
C05C - Capillary stabilizing agents
C05CA - Bioflavonoids
C05CA03 - Diosmin
Absorption
Diosmin is rapidly absorbed in the gastrointestinal tract. After a 900 mg single oral dose in a study using liquid chromatography with tandem mass spectrometry (LC-MS/MS) method, Cmax was 4.23.8 ngmL-1, Tmax was 18.79.9 hours, and AUC0~96 was 185.4166.2 ngmL-1 in healthy volunteers. Another pharmacokinetic study of 5 adults revealed a Cmax of 41794.1 ng/dL.
Route of Elimination
Pharmacokinetic data show absence of urinary elimination for diosmin and its aglycone diosmetin. Minor metabolites are found to be eliminated in the urine as glucuronic acid conjugates.
Volume of Distribution
A pharmacokinetic study of 5 adults revealed a volume of distribution of 62.17.9 L.
Degradation products of diosmin such as alkyl-phenolic acids confirm a metabolic pattern similar to that of other flavonoids.
Diosmin half-life ranges from 26 to 43 hours. One study using a liquid chromatography with tandem mass spectrometry (LC-MS/MS) method after a single 900 mg dose of diosmin demonstrated a half-life of 60.285.7 hours in healthy volunteers.
Diosmin helps to maintain circulatory system structure and function, particularly vein strength and competence. The molecular mechanism of action of diosmin has not been established. Several resources indicate that diosmin binds to the aryl hydrocarbon receptor, however clinical relevance to vascular function is unknown. One study demonstrates that oral diosmin exerts effects on the in vitro metabolism of noradrenaline by varicose veins, potentially benefitting vascular health.
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39669
Submission : 2024-04-05
Status : Active
Type : II

Certificate Number : R1-CEP 2008-274 - Rev 07
Issue Date : 2020-06-03
Type : Chemical
Substance Number : 1611
Status : Valid

Registrant Name : Sungjin Exim Co., Ltd.
Registration Date : 2021-03-23
Registration Number : 20210323-211-J-904
Manufacturer Name : HealthTech Bio Actives, SLU
Manufacturer Address : Ctra. De Zeneta 143-145 El Raiguero - La Villa 30130 Beniel (Murcia) Spain
 PMC Isochem is your partner for smart CDMOs of Intermediates, APIs, & excipients & a catalog of Intermediates & Generic APIs.
PMC Isochem is your partner for smart CDMOs of Intermediates, APIs, & excipients & a catalog of Intermediates & Generic APIs.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 17084
Submission : 2003-12-12
Status : Active
Type : II

Certificate Number : R1-CEP 2003-153 - Rev 05
Issue Date : 2019-01-25
Type : Chemical
Substance Number : 1611
Status : Valid
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 26482
Submission : 2012-09-24
Status : Inactive
Type : II

Certificate Number : R1-CEP 2013-229 - Rev 00
Issue Date : 2020-01-08
Type : Chemical
Substance Number : 1611
Status : Valid

Registrant Name : Pharmapia Co., Ltd.
Registration Date : 2025-03-14
Registration Number : 20250314-211-J-1777
Manufacturer Name : Sichuan Xieli Pharmaceutical Co., Ltd.
Manufacturer Address : No. 588, Middle Sectionof Mudan Avenue, TianpengTown, Pengzhou City, Sichuan Province, China

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 25631
Submission : 2011-12-05
Status : Inactive
Type : II

Certificate Number : R1-CEP 2011-177 - Rev 01
Issue Date : 2020-05-07
Type : Chemical
Substance Number : 1611
Status : Valid

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 19259
Submission : 2006-02-06
Status : Inactive
Type : II
Certificate Number : R0-CEP 2009-032 - Rev 01
Issue Date : 2015-03-31
Type : Chemical
Substance Number : 1611
Status : Withdrawn by EDQM F...


Certificate Number : R1-CEP 2016-202 - Rev 00
Issue Date : 2022-09-13
Type : Chemical
Substance Number : 1611
Status : Valid


Certificate Number : R1-CEP 2017-087 - Rev 00
Issue Date : 2023-06-13
Type : Chemical
Substance Number : 1611
Status : Valid


Certificate Number : CEP 2024-194 - Rev 00
Issue Date : 2024-07-09
Type : Chemical
Substance Number : 1611
Status : Valid


Certificate Number : R1-CEP 2016-173 - Rev 00
Issue Date : 2022-06-22
Type : Chemical
Substance Number : 1611
Status : Valid

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Diosmin Hesperidin Zentiva
Dosage Form : Filmtabl
Dosage Strength : 500mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Diosmin Hesperidin Zentiva
Dosage Form : Filmtabl
Dosage Strength : 500mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Diosmin Hesperidin Zentiva
Dosage Form : Filmtabl
Dosage Strength : 500mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Daflon 500
Dosage Form : Tabl
Dosage Strength : 500mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Daflon 500
Dosage Form : Tabl
Dosage Strength : 500mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : Turkey
Brand Name :
Dosage Form : TABLET
Dosage Strength : 225MG; 25MG
Packaging : 30 Tablets
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : Turkey

Regulatory Info :
Registration Country : Italy
Brand Name : Daflon
Dosage Form :
Dosage Strength : 30 Cpr Riv 500 Mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Italy
Brand Name : Daflon
Dosage Form :
Dosage Strength : 30 Cpr Riv 500 Mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Italy
Brand Name : Daflon
Dosage Form :
Dosage Strength : 30 Cpr Riv 500 Mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Italy
Brand Name : Daflon
Dosage Form :
Dosage Strength : 30 Cpr Riv 500 Mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
12
PharmaCompass offers a list of Diosmin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Diosmin manufacturer or Diosmin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Diosmin manufacturer or Diosmin supplier.
PharmaCompass also assists you with knowing the Diosmin API Price utilized in the formulation of products. Diosmin API Price is not always fixed or binding as the Diosmin Price is obtained through a variety of data sources. The Diosmin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Diosmin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Diosmin, including repackagers and relabelers. The FDA regulates Diosmin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Diosmin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Diosmin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Diosmin supplier is an individual or a company that provides Diosmin active pharmaceutical ingredient (API) or Diosmin finished formulations upon request. The Diosmin suppliers may include Diosmin API manufacturers, exporters, distributors and traders.
click here to find a list of Diosmin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Diosmin DMF (Drug Master File) is a document detailing the whole manufacturing process of Diosmin active pharmaceutical ingredient (API) in detail. Different forms of Diosmin DMFs exist exist since differing nations have different regulations, such as Diosmin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Diosmin DMF submitted to regulatory agencies in the US is known as a USDMF. Diosmin USDMF includes data on Diosmin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Diosmin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Diosmin suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Diosmin Drug Master File in Korea (Diosmin KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Diosmin. The MFDS reviews the Diosmin KDMF as part of the drug registration process and uses the information provided in the Diosmin KDMF to evaluate the safety and efficacy of the drug.
After submitting a Diosmin KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Diosmin API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Diosmin suppliers with KDMF on PharmaCompass.
A Diosmin CEP of the European Pharmacopoeia monograph is often referred to as a Diosmin Certificate of Suitability (COS). The purpose of a Diosmin CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Diosmin EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Diosmin to their clients by showing that a Diosmin CEP has been issued for it. The manufacturer submits a Diosmin CEP (COS) as part of the market authorization procedure, and it takes on the role of a Diosmin CEP holder for the record. Additionally, the data presented in the Diosmin CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Diosmin DMF.
A Diosmin CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Diosmin CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Diosmin suppliers with CEP (COS) on PharmaCompass.
A Diosmin written confirmation (Diosmin WC) is an official document issued by a regulatory agency to a Diosmin manufacturer, verifying that the manufacturing facility of a Diosmin active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Diosmin APIs or Diosmin finished pharmaceutical products to another nation, regulatory agencies frequently require a Diosmin WC (written confirmation) as part of the regulatory process.
click here to find a list of Diosmin suppliers with Written Confirmation (WC) on PharmaCompass.
Diosmin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Diosmin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Diosmin GMP manufacturer or Diosmin GMP API supplier for your needs.
A Diosmin CoA (Certificate of Analysis) is a formal document that attests to Diosmin's compliance with Diosmin specifications and serves as a tool for batch-level quality control.
Diosmin CoA mostly includes findings from lab analyses of a specific batch. For each Diosmin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Diosmin may be tested according to a variety of international standards, such as European Pharmacopoeia (Diosmin EP), Diosmin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Diosmin USP).
