


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
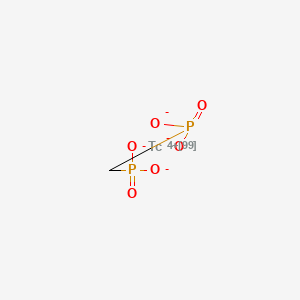

1. Tc-99m-diphosphonate
2. Technetium Diphosphonate
3. Technetium Tc 99m Diphosphonate
1. 8v3fgc4j77
2. Technetium Tc-99m Medronate Anhydrous
3. Tc-99m-diphosphonate
4. Technetium Diphosphonate
5. Tc-mdp
6. Tc-mdp Tc-99m
7. Technetium Tc 99m Diphosphonate
8. Technetium Tc-99m Diphosphonate
9. Diphosphonic Acid, Technetium-99tc Salt
10. Q27271059
11. Dioxido-oxo-(phosphonatomethyl)-lambda5-phosphane;technetium-99(4+)
12. Phosphonic Acid, Methylenebis-, Technetium(4+) Salt (1:1)
13. Phosphonic Acid, Methylenebis-, Technetium(4+)-99tc Salt (1:1)
| Molecular Weight | 270.88 g/mol |
|---|---|
| Molecular Formula | CH2O6P2Tc |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 0 |
| Exact Mass | 270.838911 g/mol |
| Monoisotopic Mass | 270.838911 g/mol |
| Topological Polar Surface Area | 126 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 129 |
| Isotope Atom Count | 1 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Radiopharmaceuticals
Compounds that are used in medicine as sources of radiation for radiotherapy and for diagnostic purposes. They have numerous uses in research and industry. (Martindale, The Extra Pharmacopoeia, 30th ed, p1161) (See all compounds classified as Radiopharmaceuticals.)


