
Synopsis
Synopsis
0
KDMF
0
VMF
0
Australia

0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Antistenocardin
2. Apo Dipyridamole
3. Apo-dipyridamole
4. Cerebrovase
5. Clridium
6. Curantil
7. Curantyl
8. Dipyramidole
9. Kurantil
10. Miosen
11. Novo Dipiradol
12. Novo-dipiradol
13. Persantin
14. Persantine
1. 58-32-2
2. Persantin
3. Dipyridamol
4. Dipyridamine
5. Persantine
6. Dipyudamine
7. Curantyl
8. Stimolcardio
9. Cardoxin
10. Kurantil
11. Stenocardil
12. Cardioflux
13. Dipiridamol
14. Dipyridan
15. Peridamol
16. Anginal
17. Apricor
18. Coribon
19. Corosan
20. Coroxin
21. Stenocardiol
22. Agilease
23. Chilcolan
24. Justpertin
25. Permiltin
26. Piroan
27. Cleridium 150
28. Coronarine
29. Gulliostin
30. Prandiol
31. Natyl
32. Prandiol 75
33. Usaf Ge-12
34. Dypyridamol
35. Dipyridamolum
36. Dypyridamole
37. 2,2',2'',2'''-((4,8-di(piperidin-1-yl)pyrimido[5,4-d]pyrimidine-2,6-diyl)bis(azanetriyl))tetraethanol
38. Ra 8
39. Ra-8
40. Nsc-515776
41. Dipyridamole (persantine)
42. Nsc 515776
43. Mls000028420
44. 2-[[2-[bis(2-hydroxyethyl)amino]-4,8-di(piperidin-1-yl)pyrimido[5,4-d]pyrimidin-6-yl]-(2-hydroxyethyl)amino]ethanol
45. Cleridium
46. 2-({6-[bis(2-hydroxyethyl)amino]-4,8-bis(piperidin-1-yl)pyrimido[5,4-d][1,3]diazin-2-yl}(2-hydroxyethyl)amino)ethan-1-ol
47. Chembl932
48. 2,6-bis(diethanolamino)-4,8-dipiperidinopyrimido(5,4-d)pyrimidine
49. Iv Persantine
50. Smr000058382
51. 64alc7f90c
52. Chebi:4653
53. Cardoxil
54. B01ac07
55. Pyrimido(5,4-d)pyrimidine, 2,6-bis(bis(2-hydroxyethyl)amino)-4,8-dipiperidino-
56. Nsc515776
57. Ethanol, 2,2',2'',2'''-[(4,8-di-1-piperidinylpyrimido[5,4-d]pyrimidine-2,6-diyl)dinitrilo]tetrakis-
58. Cas-58-32-2
59. Ncgc00015385-12
60. Coridil
61. Protangix
62. 2,6-bis(diethanolamino)-4,8-dipiperidinopyrimido[5,4-d]pyrimidine
63. Dsstox_cid_20668
64. Dsstox_rid_79531
65. Dsstox_gsid_40668
66. 2,2',2'',2'''-((4,8-di(piperidin-1-yl)pyrimido[5,4-d]pyrimidine-2,6-diyl)bis(azanetriyl))tetrakis(ethan-1-ol)
67. 2,2',2'',2'''-{[4,8-di(piperidin-1-yl)pyrimido[5,4-d]pyrimidine-2,6-diyl]dinitrilo}tetraethanol
68. Ethanol, 2,2',2'',2'''-((4,8-di-1-piperidinylpyrimido(5,4-d)pyrimidine-2,6-diyl)dinitrilo)tetrakis-
69. Dipiridamol [inn-spanish]
70. Dipyridamolum [inn-latin]
71. Permole
72. Persantine (tn)
73. (3e)-3-[[(1s,4as,8as)-decahydro-5,5,8a-trimethyl-2-methylene-1-naphthalenyl]methylene]dihydro-5-methoxy-2(3h)3-[(1e)-2-[(1s,4as,8as)-decahydro-5,5,8a-trimethyl-2-methylene-1-naphthalenyl]ethenyl]furan; (+)-coronarin E-furanone; Coronarin D Methyl Ethe
74. 2,2',2'',2'''-((4,8-di(piperidin-1-yl)pyrimido[5,4-d]-pyrimidine-2,6-diyl)bis(azanetriyl))tetraethanol
75. Sr-01000003065
76. Einecs 200-374-7
77. Brn 0068373
78. Unii-64alc7f90c
79. Dipridacot
80. Curanty
81. Prestwick_145
82. Mfcd00010555
83. Dipyridamole [usan:usp:inn:ban:jan]
84. Spectrum_001004
85. Tocris-0691
86. Opera_id_494
87. Prestwick0_000142
88. Prestwick1_000142
89. Prestwick2_000142
90. Prestwick3_000142
91. Spectrum2_000972
92. Spectrum3_000402
93. Spectrum4_000522
94. Spectrum5_000839
95. Dipyridamole [mi]
96. Lopac-d-9766
97. Dipyridamole [inn]
98. Dipyridamole [jan]
99. Upcmld-dp072
100. D 9766
101. Dipyridamole [usan]
102. Cid_3108
103. 2,2',2'',2'''-((4,8-dipiperidinopyrimido(5,4-d)pyrimidine-2,6-diyl)dinitrilo)tetraethanol
104. Dipyridamole [vandf]
105. Lopac0_000464
106. Schembl16119
107. Bspbio_000244
108. Bspbio_001554
109. Bspbio_001924
110. Dipyridamole [mart.]
111. Kbiogr_001123
112. Kbioss_001484
113. 4-26-00-03840 (beilstein Handbook Reference)
114. Mls001076306
115. Mls001333724
116. Mls002548866
117. Dipyridamole [usp-rs]
118. Dipyridamole [who-dd]
119. Divk1c_000696
120. Spectrum1500259
121. Spbio_001003
122. Spbio_002183
123. Bpbio1_000270
124. Gtpl4807
125. Dtxsid6040668
126. Thymidine,6-dihydro-6-methoxy-
127. Upcmld-dp072:001
128. Bdbm23620
129. Dipyridamole (jp17/usp/inn)
130. Hms502c18
131. Izekfcxsfnuwam-uhfffaoysa-
132. Kbio1_000696
133. Kbio2_001484
134. Kbio2_004052
135. Kbio2_006620
136. Kbio3_001144
137. Ninds_000696
138. Bcpp000256
139. Dipyridamole [orange Book]
140. Hms1568m06
141. Hms1791n16
142. Hms1920i10
143. Hms1989n16
144. Hms2089n15
145. Hms2091o18
146. Hms2095m06
147. Hms2232e19
148. Hms3259c03
149. Hms3261m10
150. Hms3266j17
151. Hms3371j03
152. Hms3402n16
153. Hms3411b03
154. Hms3655i20
155. Hms3675b03
156. Hms3712m06
157. Hms3742o03
158. Hms3867f13
159. Pharmakon1600-01500259
160. Zinc643046
161. Dipyridamole [ep Monograph]
162. Amy40468
163. Bcp26947
164. Hy-b0312
165. Dipyridamole [usp Monograph]
166. Tox21_110133
167. Tox21_500464
168. 2,6-bis(diethanolamine)-4,8-dipiperidinopyrimido[5,4-d]pyrimidine
169. Bbl027781
170. Ccg-40190
171. Nsc756743
172. S1895
173. Stl377790
174. Aggrenox Component Dipyridamole
175. Akos000509426
176. Tox21_110133_1
177. Bcp9000613
178. Db00975
179. Dipyridamole, >=98% (tlc), Powder
180. Hs-0041
181. Lp00464
182. Nc00448
183. Nsc-619103
184. Nsc-756743
185. Sdccgsbi-0050449.p005
186. Idi1_000696
187. Smp2_000208
188. Dipyridamole Component Of Aggrenox
189. Ncgc00015385-01
190. Ncgc00015385-02
191. Ncgc00015385-03
192. Ncgc00015385-04
193. Ncgc00015385-05
194. Ncgc00015385-06
195. Ncgc00015385-07
196. Ncgc00015385-08
197. Ncgc00015385-09
198. Ncgc00015385-10
199. Ncgc00015385-11
200. Ncgc00015385-13
201. Ncgc00015385-14
202. Ncgc00015385-15
203. Ncgc00015385-16
204. Ncgc00015385-17
205. Ncgc00015385-18
206. Ncgc00015385-29
207. Ncgc00023914-02
208. Ncgc00023914-04
209. Ncgc00023914-05
210. Ncgc00023914-06
211. Ncgc00023914-07
212. Ncgc00023914-08
213. Ncgc00023914-09
214. Ncgc00023914-10
215. Ncgc00023914-11
216. Ncgc00261149-01
217. 2-({6-[bis(2-hydroxyethyl)amino]-4,8-bis(piperidin-1-yl)-[1,3]diazino[5,4-d]pyrimidin-2-yl}(2-hydroxyethyl)amino)ethan-1-ol
218. 2-[[2-[bis(2-hydroxyethyl)amino]-4,8-bis(1-piperidyl)pyrimido[5,4-d]pyrimidin-6-yl]-(2-hydroxyethyl)amino]ethanol
219. Ac-18100
220. Ac-30804
221. Ethanol, 2,2',2'',2'''-((4,8-dipiperidinopyrimido(5,4-d)pyrimidine-2,6-diyl)dinitrilo)tetra-
222. Ethanol,2,2',2'',2'''-[(4,8-di-1-piperidinylpyrimido[5,4-d]pyrimidine-2,6-diyl)dinitrilo]tetrakis-
223. Nci60_005689
224. Sbi-0050449.p004
225. 2,8-dipiperidinopyrimido[5,4-d]pyrimidine
226. Ab00051974
227. B1933
228. Eu-0100464
229. Ft-0603242
230. Sw196456-3
231. En300-70723
232. Bim-0050449.0001
233. D00302
234. O10551
235. Ab00051974-18
236. Ab00051974-19
237. Ab00051974_20
238. Ab00051974_21
239. A828156
240. A831828
241. Q419374
242. Sr-01000003065-2
243. Sr-01000003065-4
244. Sr-01000003065-5
245. Sr-01000003065-7
246. W-105400
247. Brd-k86301799-001-04-1
248. Brd-k86301799-001-19-9
249. Brd-k86301799-001-24-9
250. Z1259192074
251. Dipyridamole, British Pharmacopoeia (bp) Reference Standard
252. Dipyridamole, European Pharmacopoeia (ep) Reference Standard
253. 2,6-bis(diethanolamino)-4,8-dipiperidinopyrimido-[5,4-d]pyrimidin
254. Dipyridamole, United States Pharmacopeia (usp) Reference Standard
255. Pyrimido(5, 2,6-bis[bis(2-hydroxyethyl)amino]-4,8-diperidino-
256. 2,2'',2'''-[4,8-dipiperidinopyrimido[5,4-d]pyrimidine-2,6-diyl]dinitrilotetraethanol
257. 2,2',2'',2'''-(4,8-dipiperidinopyrimido(5,4-d)pyrimidine-2,6-diyl)dinitrilotetraethanol
258. 2-[[2-(bis(2-hydroxyethyl)amino)-4,8-di(piperidin-1-yl)pyrimido[6,5-e]pyrimidin-
259. Dipyridamole For Peak Identification, European Pharmacopoeia (ep) Reference Standard
260. Ethanol,2',2'',2'''-(4,8-dipiperidinopyrimido[5,4-d]pyrimidine-2,6-diyldinitrilo)tetra-
261. Wln: T66 Bn Dn Gn Inj Ccn Hcn E- At6ntj B2q F2q& J- At6ntj B2q F2q
262. 2,2',2'',2'''-(4,8-di(piperidin-1-yl)pyrimido[5,4-d]pyrimidine-2,6-diyl)bis(azanetriyl)tetraethanol
263. 2,2',2'',2'''-[(4,8-dipiperidin-1-ylpyrimido[5,4-d]pyrimidine-2,6-diyl)dinitrilo]tetraethanol
264. 2,2',2'',2'''-[(4,8-dipiperidinylpyrimido[5,4-d]pyrimidine-2,6-diyl)dinitrilo]tetraethanol
265. 2,2',2'',2'''-[(4,8-dipiperidinylpyrimido[5,4-d]pyrimidine-2,6-diyl)dinitrilo]tetrakisethanol
266. 2-[[2-[bis(2-hydroxyethyl)amino]-4,8-bis(1-piperidinyl)-6-pyrimido[5,4-d]pyrimidinyl]-(2-hydroxyethyl)amino]ethanol
267. Ethanol, 2,2',2'',2'''-(4,8-dipiperidinopyrimido(5,4-d)pyrimidine-2,6-diyldinitrilo)tetra-
268. Ethanol, 2,2',2',2'''-((4,8-di-1-iperidinylpyrimido(5,4-d)pyrimidine-2,6-diyl)dinitrilo)tetrakis-
269. Ethanol,2',2'',2'''-[(4,8-di-1-piperidinylpyrimido[5,4-d]pyrimidine-2,6-diyl)dinitrilo]tetrakis-
270. Ethanol,2',2'',2'''-[(4,8-dipiperidinopyrimido[5,4-d]pyrimidine-2,6-diyl)dinitrilo]tetra-
271. H9f
| Molecular Weight | 504.6 g/mol |
|---|---|
| Molecular Formula | C24H40N8O4 |
| XLogP3 | 0.7 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 12 |
| Exact Mass | 504.31725179 g/mol |
| Monoisotopic Mass | 504.31725179 g/mol |
| Topological Polar Surface Area | 145 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 561 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Aggrenox |
| PubMed Health | Aspirin/Dipyridamole (By mouth) |
| Drug Classes | Platelet Aggregation Inhibitor, Phosphodiesterase Inhibitor/Salicylate, Aspirin Combination |
| Active Ingredient | Aspirin; dipyridamole |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 200mg; 25mg |
| Market Status | Prescription |
| Company | Boehringer Ingelheim |
| 2 of 6 | |
|---|---|
| Drug Name | Dipyridamole |
| PubMed Health | Dipyridamole |
| Drug Classes | Diagnostic Agent, Cardiac Function, Platelet Aggregation Inhibitor |
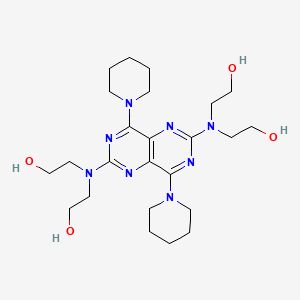
| Drug Label | Dipyridamole is a coronary vasodilator described as 2,6 bis-(diethanolamino)-4,8 dipiperidino-pyrimido-(5,4-d) pyrimidine. The structural formula is:C24H40N8O4MW 504.63Dipyri... |
| Active Ingredient | Dipyridamole |
| Dosage Form | Injectable; Tablet |
| Route | Injection; Oral |
| Strength | 75mg; 5mg/ml; 25mg; 50mg |
| Market Status | Prescription |
| Company | Bedford; Fresenius Kabi Usa; Hikma Maple; Lannett; Purepac Pharm; Zydus Pharms Usa; Impax Labs; Murty Pharms; Barr |
| 3 of 6 | |
|---|---|
| Drug Name | Persantine |
| PubMed Health | Dipyridamole |
| Drug Classes | Diagnostic Agent, Cardiac Function, Platelet Aggregation Inhibitor |
| Drug Label | PERSANTINE (dipyridamole USP) is a platelet inhibitor chemically described as 2,2',2",2"'-[(4,8- Dipiperidinopyrimido[5,4-d]pyrimidine-2,6-diyl)dinitrilo]-tetraethanol. It has the following structural formula:Dipyridamole is an odorles |
| Active Ingredient | Dipyridamole |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 75mg; 50mg |
| Market Status | Prescription |
| Company | Boehringer Ingelheim |
| 4 of 6 | |
|---|---|
| Drug Name | Aggrenox |
| PubMed Health | Aspirin/Dipyridamole (By mouth) |
| Drug Classes | Platelet Aggregation Inhibitor, Phosphodiesterase Inhibitor/Salicylate, Aspirin Combination |
| Active Ingredient | Aspirin; dipyridamole |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 200mg; 25mg |
| Market Status | Prescription |
| Company | Boehringer Ingelheim |
| 5 of 6 | |
|---|---|
| Drug Name | Dipyridamole |
| PubMed Health | Dipyridamole |
| Drug Classes | Diagnostic Agent, Cardiac Function, Platelet Aggregation Inhibitor |
| Drug Label | Dipyridamole is a coronary vasodilator described as 2,6 bis-(diethanolamino)-4,8 dipiperidino-pyrimido-(5,4-d) pyrimidine. The structural formula is:C24H40N8O4MW 504.63Dipyri... |
| Active Ingredient | Dipyridamole |
| Dosage Form | Injectable; Tablet |
| Route | Injection; Oral |
| Strength | 75mg; 5mg/ml; 25mg; 50mg |
| Market Status | Prescription |
| Company | Bedford; Fresenius Kabi Usa; Hikma Maple; Lannett; Purepac Pharm; Zydus Pharms Usa; Impax Labs; Murty Pharms; Barr |
| 6 of 6 | |
|---|---|
| Drug Name | Persantine |
| PubMed Health | Dipyridamole |
| Drug Classes | Diagnostic Agent, Cardiac Function, Platelet Aggregation Inhibitor |
| Drug Label | PERSANTINE (dipyridamole USP) is a platelet inhibitor chemically described as 2,2',2",2"'-[(4,8- Dipiperidinopyrimido[5,4-d]pyrimidine-2,6-diyl)dinitrilo]-tetraethanol. It has the following structural formula:Dipyridamole is an odorles |
| Active Ingredient | Dipyridamole |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 75mg; 50mg |
| Market Status | Prescription |
| Company | Boehringer Ingelheim |
For as an adjunct to coumarin anticoagulants in the prevention of postoperative thromboembolic complications of cardiac valve replacement and also used in prevention of angina.
FDA Label
Dipyridamole, a non-nitrate coronary vasodilator that also inhibits platelet aggregation, is combined with other anticoagulant drugs, such as warfarin, to prevent thrombosis in patients with valvular or vascular disorders. Dipyridamole is also used in myocardial perfusion imaging, as an antiplatelet agent, and in combination with aspirin for stroke prophylaxis.
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Phosphodiesterase Inhibitors
Compounds which inhibit or antagonize the biosynthesis or actions of phosphodiesterases. (See all compounds classified as Phosphodiesterase Inhibitors.)
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AC - Platelet aggregation inhibitors excl. heparin
B01AC07 - Dipyridamole
Absorption
70%
Route of Elimination
Dipyridamole is metabolized in the liver to the glucuronic acid conjugate and excreted with the bile.
Volume of Distribution
1 to 2.5 L/kg
Clearance
2.3-3.5 mL/min/kg
hepatic
40 minutes
Dipyridamole likely inhibits both adenosine deaminase and phosphodiesterase, preventing the degradation of cAMP, an inhibitor of platelet function. This elevation in cAMP blocks the release of arachidonic acid from membrane phospholipids and reduces thromboxane A2 activity. Dipyridamole also directly stimulates the release of prostacyclin, which induces adenylate cyclase activity, thereby raising the intraplatelet concentration of cAMP and further inhibiting platelet aggregation.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
66
PharmaCompass offers a list of Dipyridamole API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Dipyridamole manufacturer or Dipyridamole supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Dipyridamole manufacturer or Dipyridamole supplier.
PharmaCompass also assists you with knowing the Dipyridamole API Price utilized in the formulation of products. Dipyridamole API Price is not always fixed or binding as the Dipyridamole Price is obtained through a variety of data sources. The Dipyridamole Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Dipyridamole manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Dipyridamole, including repackagers and relabelers. The FDA regulates Dipyridamole manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Dipyridamole API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Dipyridamole manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Dipyridamole supplier is an individual or a company that provides Dipyridamole active pharmaceutical ingredient (API) or Dipyridamole finished formulations upon request. The Dipyridamole suppliers may include Dipyridamole API manufacturers, exporters, distributors and traders.
click here to find a list of Dipyridamole suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Dipyridamole DMF (Drug Master File) is a document detailing the whole manufacturing process of Dipyridamole active pharmaceutical ingredient (API) in detail. Different forms of Dipyridamole DMFs exist exist since differing nations have different regulations, such as Dipyridamole USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Dipyridamole DMF submitted to regulatory agencies in the US is known as a USDMF. Dipyridamole USDMF includes data on Dipyridamole's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Dipyridamole USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Dipyridamole suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Dipyridamole Drug Master File in Japan (Dipyridamole JDMF) empowers Dipyridamole API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Dipyridamole JDMF during the approval evaluation for pharmaceutical products. At the time of Dipyridamole JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Dipyridamole suppliers with JDMF on PharmaCompass.
A Dipyridamole CEP of the European Pharmacopoeia monograph is often referred to as a Dipyridamole Certificate of Suitability (COS). The purpose of a Dipyridamole CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Dipyridamole EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Dipyridamole to their clients by showing that a Dipyridamole CEP has been issued for it. The manufacturer submits a Dipyridamole CEP (COS) as part of the market authorization procedure, and it takes on the role of a Dipyridamole CEP holder for the record. Additionally, the data presented in the Dipyridamole CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Dipyridamole DMF.
A Dipyridamole CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Dipyridamole CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Dipyridamole suppliers with CEP (COS) on PharmaCompass.
A Dipyridamole written confirmation (Dipyridamole WC) is an official document issued by a regulatory agency to a Dipyridamole manufacturer, verifying that the manufacturing facility of a Dipyridamole active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Dipyridamole APIs or Dipyridamole finished pharmaceutical products to another nation, regulatory agencies frequently require a Dipyridamole WC (written confirmation) as part of the regulatory process.
click here to find a list of Dipyridamole suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Dipyridamole as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Dipyridamole API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Dipyridamole as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Dipyridamole and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Dipyridamole NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Dipyridamole suppliers with NDC on PharmaCompass.
Dipyridamole Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Dipyridamole GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Dipyridamole GMP manufacturer or Dipyridamole GMP API supplier for your needs.
A Dipyridamole CoA (Certificate of Analysis) is a formal document that attests to Dipyridamole's compliance with Dipyridamole specifications and serves as a tool for batch-level quality control.
Dipyridamole CoA mostly includes findings from lab analyses of a specific batch. For each Dipyridamole CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Dipyridamole may be tested according to a variety of international standards, such as European Pharmacopoeia (Dipyridamole EP), Dipyridamole JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Dipyridamole USP).
