
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
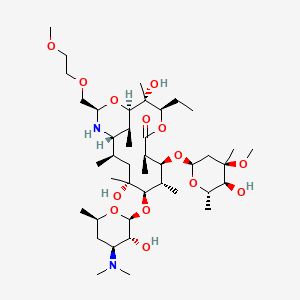

1. (9s)-9-deoxo-11-deoxy-9,11-(imino((1r)-2-(2-methoxyethoxy)ethylidene)oxy)erythromycin
2. Dynabac
3. Ly 237216
4. Ly-237216
5. Nortron
1. 62013-04-1
2. Dynabac
3. Dirithromycine
4. Dirithromycinum
5. Diritromicina
6. Ly 237216
7. Ly-237216
8. (9s)-9-deoxo-11-deoxy-9,11-(imino((1r)-2-(2-methoxyethoxy)ethylidene)oxy)erythromycin
9. Mls000028564
10. Smr000058954
11. Mls001074061
12. Chebi:474014
13. 1801d76stl
14. Nsc-758672
15. Erythromycin, 9-deoxo-11-deoxy-9,11-(imino(2-(2-methoxyethoxy)ethylidene)oxy)-, (9s(r))-
16. Ly237216
17. (1r,2r,3r,6r,7s,8s,9r,10r,12r,13s,15r,17s)-3-ethyl-2,10-dihydroxy-15-[(2-methoxyethoxy)methyl]-2,6,8,10,12,17-hexamethyl-5-oxo-9-{[3,4,6-trideoxy-3-(dimethylamino)-beta-d-xylo-hexopyranosyl]oxy}-4,16-dioxa-14-azabicyclo[11.3.1]heptadec-7-yl 2,6-dideoxy-3-c-methyl-3-o-methyl-alpha-l-ribo-hexopyranoside
18. (1r,2r,3r,6r,7s,8s,9r,10r,12r,13s,15r,17s)-9-(((2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2h-pyran-2-yl)oxy)-3-ethyl-2,10-dihydroxy-7-(((2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2h-pyran-2-yl)oxy)-15-((2-methoxyethoxy)methyl)-2,6,8,10,12,17-hexamethyl-4,16-dioxa-14-azabicyclo[11.3.1]heptadecan-5-one
19. Dirithromycine [french]
20. Dirithromycinum [latin]
21. Diritromicina [spanish]
22. Divitross
23. Noriclan
24. Valodin
25. Unii-1801d76stl
26. Ccris 9506
27. Ncgc00178154-03
28. Di0
29. Dynabac (tn)
30. Dirithromycin [usan:usp:inn:ban]
31. Mfcd00865041
32. Spectrum_001476
33. Specplus_000868
34. Opera_id_1686
35. Prestwick3_000557
36. Spectrum2_001655
37. Spectrum3_001630
38. Spectrum4_000597
39. Spectrum5_000715
40. Dirithromycin (usp/inn)
41. Dirithromycin [mi]
42. Dirithromycin [inn]
43. Dirithromycin [usan]
44. Bspbio_000633
45. Bspbio_003299
46. Dirithromycin [vandf]
47. Kbiogr_001094
48. Kbioss_001956
49. Dirithromycin [mart.]
50. Divk1c_006964
51. Spectrum1504144
52. Spbio_001809
53. Dirithromycin [usp-rs]
54. Dirithromycin [who-dd]
55. Bpbio1_000697
56. Chembl1237072
57. Chembl3039471
58. Bdbm59397
59. Cid_6473883
60. Kbio1_001908
61. Kbio2_001956
62. Kbio2_004524
63. Kbio2_007092
64. Kbio3_002519
65. Ase 136
66. Ase-136
67. Hms1922d13
68. Hms2093a06
69. Hms2096p15
70. Hms2233m04
71. Hms3713p15
72. Dirithromycin [ep Impurity]
73. Dirithromycin [orange Book]
74. As-e 136
75. Dirithromycin [ep Monograph]
76. Dirithromycin [usp Impurity]
77. Ccg-39072
78. Zinc96095661
79. Akos025310151
80. Db00954
81. Nsc 758672
82. Ncgc00178154-01
83. Ncgc00178154-02
84. Sbi-0052687.p002
85. Ab00513862
86. D03865
87. T72716
88. Ab00053192_13
89. 013d041
90. A833532
91. Sr-01000721832
92. Sr-05000001888
93. Q1989071
94. Sr-01000721832-3
95. Sr-05000001888-1
96. (9s)-9-deoxo-11-deoxy-9,11-[imino[2-(2-methoxyethoxy)ethylidene]erythromycin
97. (1r,2r,3r,6r,7s,8s,9r,10r,12r,13s,15r,17s)-3-ethyl-2,10-dihydroxy-15-[(2-methoxyethoxy)methyl]-2,6,8,10,12,17-hexamethyl-5-oxo-9-{[3,4,6-trideoxy-3-(dimethylamino)-beta-d-xylo-hexopyranosyl]oxy}-4,16-dioxa-14-azabicyclo[11.3.1]heptadecan-7-yl 2,6-dideoxy-3-c-methyl-3-o-methyl-alpha-l-ribo-hexopyranoside
98. (1r,2r,3r,6r,7s,8s,9r,10r,12r,13s,15r,17s)-9-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-3-ethyl-2,10-dihydroxy-7-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-15-(2-methoxyethoxymethyl)-2,6,8,10,12,17-hexamethyl-4,16-dioxa-14-azabicyclo[11.3.1]heptadecan-5-one
99. (1s,2r,3r,6r,7s,8s,9r,10r,12r,13s,15r,17s)-9-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-3-ethyl-2,10-dihydroxy-7-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-15-(2-methoxyethoxymethyl)-2,6,8,10,12,17-hexamethyl-4,16-dioxa-14-azabicyclo[11.3.1]heptadecan-5-one
100. (1s,2r,4r,5r,6s,7s,8r,11r,12r,13r,15r,17s)-5-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-11-ethyl-4,12-dihydroxy-7-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyl-tetrahydropyran-2-yl]oxy-15-(2-methoxyethoxymethyl)-2,4,6,8,12,17-hexamethyl-10,14-dioxa-16-azabicyclo[11.3.1]heptadecan-9-one
101. (1s,2r,4r,5r,6s,7s,8r,11r,12r,13r,15r,17s)-5-[(2s,3r,4s,6r)-4-(dimethylamino)-6-methyl-3-oxidanyl-oxan-2-yl]oxy-11-ethyl-7-[(2r,4r,5s,6s)-4-methoxy-4,6-dimethyl-5-oxidanyl-oxan-2-yl]oxy-15-(2-methoxyethoxymethyl)-2,4,6,8,12,17-hexamethyl-4,12-bis(oxidanyl
102. (1s,2r,4r,5r,6s,7s,8r,11r,12r,13r,15r,17s)-5-[[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyl-2-oxanyl]oxy]-11-ethyl-4,12-dihydroxy-7-[[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyl-2-oxanyl]oxy]-15-(2-methoxyethoxymethyl)-2,4,6,8,12,17-hexamethyl-10
| Molecular Weight | 835.1 g/mol |
|---|---|
| Molecular Formula | C42H78N2O14 |
| XLogP3 | 4.2 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 16 |
| Rotatable Bond Count | 12 |
| Exact Mass | 834.54530517 g/mol |
| Monoisotopic Mass | 834.54530517 g/mol |
| Topological Polar Surface Area | 196 Ų |
| Heavy Atom Count | 58 |
| Formal Charge | 0 |
| Complexity | 1300 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 20 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of the following mild-to-moderate infections caused by susceptible strains of microorganisms: acute bacterial exacerbations of chronic bronchitis, secondary bacterial infection of acute bronchitis, community-acquired pneumonia, pharyngitis/tonsilitis, and uncomplicated skin and skin structure infections.
Dirithromycin is a pro-drug which is converted non-enzymatically during intestinal absorption into the microbiologically active moiety erythromycylamine. Erythromycylamine exerts its activity by binding to the 50S ribosomal subunits of susceptible mircoorganisms resulting in inhibition of protein synthesis. Dirithromycin/erythromycylamine has been shown to be active against most strains of the following microorganisms both in vitro and in clinical infections: Staphylococcus aureus (methicillin-susceptible strains only), Streptococcus pneumoniae, Streptococcus pyogenes, Haemophilus influenzae, Legionella pneumophila, Moraxella catarrhalis, and Mycoplasma pneumoniae.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01F - Macrolides, lincosamides and streptogramins
J01FA - Macrolides
J01FA13 - Dirithromycin
Absorption
Oral dirithromycin is rapidly absorbed, with an absolute bioavailability of approximately 10%. Dietary fat has little or no effect on the bioavailability of dirithromycin.
Dirithromycin is converted by nonenzymatic hydrolysis during absorption to the active compound, erythromycylamine. Sixty to 90% of a dose is hydrolyzed to erythromycylamine within 35 minutes after dosing, and conversion is nearly complete after 1.5 hours. Erythromycylamine undergoes little or no hepatic biotransformation. No other metabolites of dirithromycin have been detected in the serum.
The mean plasma half-life of erythromycylamine was estimated to be about 8 h (2 to 36 h), with a mean urinary terminal elimination half-life of about 44 h (16 to 65 h) in patients with normal renal function.
Dirithromycin prevents bacteria from growing, by interfering with their protein synthesis. Dirithromycin binds to the 50S subunit of the 70S bacterial ribosome, and thus inhibits the translocation of peptides. Dirithromycin has over 10 times higher affinity to the subunit 50S than erythromycin. In addition, dirithromycin binds simultaneously in to two domains of 23S RNA of the ribosomal subunit 50S, where older macrolides bind only in one. Dirithromycin can also inhibit the formation of ribosomal subunits 50S and 30S.
Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
24
PharmaCompass offers a list of Dirithromycin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Dirithromycin manufacturer or Dirithromycin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Dirithromycin manufacturer or Dirithromycin supplier.
PharmaCompass also assists you with knowing the Dirithromycin API Price utilized in the formulation of products. Dirithromycin API Price is not always fixed or binding as the Dirithromycin Price is obtained through a variety of data sources. The Dirithromycin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Dirithromycin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Dirithromycin, including repackagers and relabelers. The FDA regulates Dirithromycin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Dirithromycin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Dirithromycin supplier is an individual or a company that provides Dirithromycin active pharmaceutical ingredient (API) or Dirithromycin finished formulations upon request. The Dirithromycin suppliers may include Dirithromycin API manufacturers, exporters, distributors and traders.
click here to find a list of Dirithromycin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Dirithromycin DMF (Drug Master File) is a document detailing the whole manufacturing process of Dirithromycin active pharmaceutical ingredient (API) in detail. Different forms of Dirithromycin DMFs exist exist since differing nations have different regulations, such as Dirithromycin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Dirithromycin DMF submitted to regulatory agencies in the US is known as a USDMF. Dirithromycin USDMF includes data on Dirithromycin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Dirithromycin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Dirithromycin suppliers with USDMF on PharmaCompass.
Dirithromycin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Dirithromycin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Dirithromycin GMP manufacturer or Dirithromycin GMP API supplier for your needs.
A Dirithromycin CoA (Certificate of Analysis) is a formal document that attests to Dirithromycin's compliance with Dirithromycin specifications and serves as a tool for batch-level quality control.
Dirithromycin CoA mostly includes findings from lab analyses of a specific batch. For each Dirithromycin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Dirithromycin may be tested according to a variety of international standards, such as European Pharmacopoeia (Dirithromycin EP), Dirithromycin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Dirithromycin USP).
