
Synopsis
Synopsis
0
KDMF
0
VMF
0
FDF
0
Australia

0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
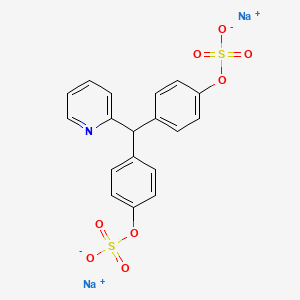

1. 4,4'-(2-picolylidene)bisphenylsulfuric Acid
2. 4,4'-(picoliliden)-bis-phenylsulphate
3. Laxoberal
4. Picolax
5. Picoprep
6. Picosulfate Sodium
7. Picosulfol
1. 10040-45-6
2. Picosulfate Sodium
3. Laxoberal
4. Laxoberon
5. Sodium Picosulphate
6. Evacuol
7. Picolax
8. Neopax
9. Guttalax-fher
10. Pico-salax
11. Da-1773
12. 4,4'-(2-picolylidene)bis(phenylsulfuric Acid) Disodium Salt
13. La 391
14. Picosulfate Sodium Anhydrous
15. Sodium Picosulfate Anhydrous
16. Anhydrous Sodium Picosulfate
17. Da 1773
18. Sodium Picosulphate Anhydrous
19. Picosulfol
20. Guttalax
21. Rapilax
22. Chebi:32147
23. 4,4'-(2-pyridylmethylene)diphenol Bis(hydrogen Sulfate) Disodium Salt
24. Vw106606y8
25. Laxidogol
26. Evanol
27. Disodium;[4-[pyridin-2-yl-(4-sulfonatooxyphenyl)methyl]phenyl] Sulfate
28. Sodium Picosulfate 100 Microg/ml In Acetonitrile
29. Picosulfate Sodium Hydrate
30. Picoprep
31. Natrii Picosulfas
32. Sodium Picosulphate Monohydrate
33. Picosulfato Sodico
34. Disodium 4,4'-(2-pyridylmethylene)-di(phenyl Sulphate);disodium 4,4'-(2-pyridylmethylene)-di(phenyl Sulphate);sodium Picosulfate
35. Natrii Picosulfas [inn-latin]
36. Picosulfate De Sodium
37. La-391
38. Picosulfato Sodico [inn-spanish]
39. Ncgc00182711-01
40. Picosulfate De Sodium [inn-french]
41. Sodium Picosulfate [inn:ban:jan]
42. Einecs 233-120-9
43. 2-picolylidenebis(p-phenyl Sodium Sulfate)
44. Natriumpicosulfat
45. Unii-vw106606y8
46. Guttalax;laxoberon
47. Disodium 4,4'-disulfoxydiphenyl-(2-pyridyl)methane
48. Bisacodyl Impurity D
49. 4-methyl-2-pentenoicacid
50. 4,4'-(2-pyridylmethylene)diphenolbis(hydrogen Sulfate) (ester) Disodium Salt
51. 4,4'-(2-pyridinylmethylene)bisphenol Bis(hydrogen Sulfate) (ester) Disodium Salt
52. Dsstox_cid_28589
53. Dsstox_rid_82860
54. Dsstox_gsid_48663
55. Schembl346436
56. Picosulfate Sodium [mi]
57. Chembl1697768
58. Dtxsid7048663
59. Sodium Picosulfate [inn]
60. Picosulfate For System Suitability
61. Hms3652k17
62. Bcp11522
63. Tox21_113026
64. Mfcd00867640
65. S4020
66. 4,4'-(2-pyridinylmethylene)bisphenol 1,1'-bis(hydrogen Sulfate) Sodium Salt
67. Phenol, 4,4'-(2-pyridylmethylene)bis-, Bis(hydrogen Sulfate), Disodium Salt
68. Ccg-269559
69. Ac-31735
70. As-15785
71. Cas-10040-45-6
72. Ft-0673898
73. S0936
74. Sw219183-1
75. C71029
76. Q410265
77. Sodium (pyridin-2-ylmethylene)bis(4,1-phenylene) Bis(sulfate)
78. Sodium 4,4'-(pyridin-2-ylmethylene)bis(4,1-phenylene) Disulfate
79. 4,4'-(2-pyridylmethylene)diphenol Bis(hydrogen Sulphate) Disodium Salt
| Molecular Weight | 481.4 g/mol |
|---|---|
| Molecular Formula | C18H13NNa2O8S2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 5 |
| Exact Mass | 480.98779728 g/mol |
| Monoisotopic Mass | 480.98779728 g/mol |
| Topological Polar Surface Area | 163 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 633 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Cathartics
Agents that are used to stimulate evacuation of the bowels. (See all compounds classified as Cathartics.)
A - Alimentary tract and metabolism
A06 - Drugs for constipation
A06A - Drugs for constipation
A06AB - Contact laxatives
A06AB08 - Sodium picosulfate
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 27627
Submission : 2013-10-08
Status : Active
Type : II

Certificate Number : CEP 2014-020 - Rev 02
Issue Date : 2024-09-20
Type : Chemical
Substance Number : 1031
Status : Valid

Date of Issue : 2022-08-29
Valid Till : 2025-07-26
Written Confirmation Number : WC-0180
Address of the Firm :

NDC Package Code : 58793-006
Start Marketing Date : 2015-07-01
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 23477
Submission : 2010-01-18
Status : Active
Type : II

Registration Number : 217MF11185
Registrant's Address : Via Curiel 34, 20067 Paulo, Milano, ITALY
Initial Date of Registration : 2005-12-12
Latest Date of Registration : --

NDC Package Code : 12828-0081
Start Marketing Date : 2011-01-19
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : Reviewed
Rev. Date : 2014-06-06
Pay. Date : 2013-11-15
DMF Number : 27646
Submission : 2013-10-17
Status : Inactive
Type : II

Date of Issue : 2022-09-16
Valid Till : 2025-07-22
Written Confirmation Number : WC-0136n
Address of the Firm :

NDC Package Code : 61876-0720
Start Marketing Date : 2014-01-07
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


Registration Number : 217MF10037
Registrant's Address : 4-8-2 Nihonbashi Honcho, Chuo-ku, Tokyo
Initial Date of Registration : 2005-05-20
Latest Date of Registration : --


Certificate Number : R1-CEP 2003-068 - Rev 00
Issue Date : 2009-11-04
Type : Chemical
Substance Number : 1031
Status : Valid

Registration Number : 220MF10132
Registrant's Address : S. S. 11 Padana Superiore,N. 8 24040 Fornovo San Giovanni
Initial Date of Registration : 2008-05-20
Latest Date of Registration : --


Certificate Number : CEP 2020-021 - Rev 02
Issue Date : 2024-07-15
Type : Chemical
Substance Number : 1031
Status : Valid

Date of Issue : 2018-01-01
Valid Till : 2021-01-01
Written Confirmation Number : WC-0409
Address of the Firm :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
About the Company : LGM Pharma is a global leader in sourcing hard-to-find APIs and intermediates for the pharmaceutical and biotech industries. LGM is also a full service CDMO providing formulation, ...
 Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
About the Company : Established in 2012, Nuray Chemicals Pvt Ltd is an API manufacturer for highly regulated markets. Its manufacturing facility with state-of-the-art R&D is located near Chennai in th...
About the Company : Jai Radhe Sales was founded in 1999 as an out-of-the-box distribution firm specializing in the global supply of high-quality pharmaceutical ingredients. The firm provides complete ...
About the Company : We have been supporting the pharmaceutical and biopharmaceutical industries with drug development for over 30 years, assisting our clients in developing and bringing new drugs to m...

About the Company : Guangzhou Tosun Pharmaceutical was founded in 1999, which mainly focuses on importation & exportation of Active Pharmaceutical Ingrediants, Chemical Raw Materials, Intermediate, Ex...

About the Company : We are a renowned pharma player having a wide range of quality products and specialise in manufacturing Active Pharmaceutical Ingredients (API) and Drug Intermediates (DI’s). The...

About the Company : Indian Pharmaceutical Company, Precise Group is emerging as a global player in several verticals in healthcare. The Mumbai based drug manufacturing company’s strength lies in a s...

About the Company : Rakshit is an integrated API manufacturing company. Established in 2000. Today Rakshit has emerged a dependable and strong manufacturer of key APIs & intermediates for global marke...

About the Company : Shreepati Pharmaceuticals is an ISO-9001 : 2015 and GMP certified company. We are a leading manufacturer and exporter of raw materials for the pharmaceutical industry. Our range i...

About the Company : Adapting quickly to the ever-evolving demands, we have emerged as one of the fastest growing organization in the pharmaceutical industry across the globe. With our headquarters loc...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?