
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
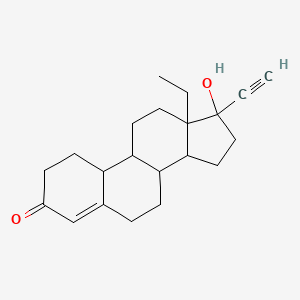

1. 18,19-dinorpregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-, (17alpha)-(+-)-
2. Dl Norgestrel
3. Dl-norgestrel
4. Neogest
5. Ovrette
6. Postinor
7. Wy 3707
8. Wy-3707
9. Wy3707
1. Methylnorethindrone
2. Ld Norgestrel
3. Norgestrelum
4. Monovar
5. Neogest
6. Ovrette
7. 17-ethynyl-17-hydroxy-18a-homoestr-4-en-3-one
8. Dl-norgestrel
9. Wy 3707
10. 13-ethyl-17-hydroxy-18,19-dinorpregn-5(10)-en-20-yn-3-one
11. Fh 122-a
12. Sh 850
13. 6533-00-2
14. Sh 70850
15. .alpha.-norgestrel
16. (.+/-.)-norgestrel
17. Oprea1_224559
18. Schembl691861
19. Chebi:7630
20. Component Of Stediril (salt/mix)
21. Act06728
22. Bcp21999
23. Akos015960898
24. Ac-6144
25. Db09389
26. Ncgc00249623-01
27. As-13043
28. Ft-0656076
29. Ft-0665638
30. L000895
31. J-012870
32. Dl-13-.beta.-ethyl-17-.alpha.-ethynyl-19-nortestosterone
33. (.+/-.)-13-ethyl-17.alpha.-hydroxy-18,19-dinorpregn-4-en-20-yn-3-one
34. Dl-13-.beta.-ethyl-17-.alpha.-ethynyl-17-.beta.-hydroxygon-4-en-3-one
35. (.+/-.)-13-ethyl-17-hydroxy-18,19-dinor-17-.alpha.-pregn-4-en-20-yn-3-one
36. 18,19-dinor-17-.alpha.-pregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-, (.+/-.)-
37. 18,19-dinorpregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-, (17.alpha.)-(.+/-.)-
38. 13-ethyl-17-hydroxy-18,19-dinor-17 Alpha -pregn-5(10)-en-20-yn-3-one(levonorgestrel Impurity B)
39. 13-ethyl-17-hydroxy-18,19-dinor-17alpha-pregn-5(10)-en-20-yn-3-one(levonorgestrel Impurity B)
| Molecular Weight | 312.4 g/mol |
|---|---|
| Molecular Formula | C21H28O2 |
| XLogP3 | 3.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 312.208930132 g/mol |
| Monoisotopic Mass | 312.208930132 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 609 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 6 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Contraceptives, Oral, Synthetic; Progestational Hormones, Synthetic
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Low-ogestrel (norgestrel and ethinyl estradiol tablets) is indicated for the prevention of pregnancy in women who elect to use this product as a method of contraception. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information Low-ogestrel (Norgestrel and Ethinyl Estradiol) (March 2007). Available from, as of March 29, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4626
/Cyclo-Progynova is indicated as/ hormone replacement therapy (HRT) for estrogen deficiency symptoms in perimenopausal and postmenopausal women.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Cyclo-Progynova (Last updated March 2011). Available from, as of March 25, 2011: https://www.medicines.org.uk/EMC/medicine/9159/SPC/Cyclo-Progynova+2mg/
/Cyclo-Progynova is indicated for/ prevention of osteoporosis in postmenopausal women at high risk of future fractures who are intolerant of, or contraindicated for, other medicinal products approved for the prevention of osteoporosis.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Cyclo-Progynova (Last updated March 2011). Available from, as of March 25, 2011: https://www.medicines.org.uk/EMC/medicine/9159/SPC/Cyclo-Progynova+2mg/
Norgestrel ... /is/ indicated for the prevention of pregnancy. Progestin-only oral contraceptives are also called minipills and progestin-only oral pills (POPs). /Former/
USP. Convention. USPDI - Drug Information for the Health Care Professional. 20th ed. Volume I. Micromedex, Inc. Englewood, CO., 2000. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2567
Cigarette smoking increases the risk of serious cardiovascular side effects from oral contraceptive use. This risk increases with age and with heavy smoking (15 or more cigarettes per day) and is quite marked in women over 35 years of age. Women who use oral contraceptives should be strongly advised not to smoke.
US Natl Inst Health; DailyMed. Current Medication Information Low-ogestrel (Norgestrel and Ethinyl Estradiol) (March 2007). Available from, as of March 29, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4626
The use of oral contraceptives is associated with increased risks of several serious conditions including myocardial infarction, thromboembolism, stroke, hepatic neoplasia, and gallbladder disease, although the risk of serious morbidity or mortality is very small in healthy women without underlying risk factors. The risk of morbidity and mortality increases significantly in the presence of other underlying risk factors such as hypertension, hyperlipidemias, hypercholesterolemia, obesity and diabetes.
US Natl Inst Health; DailyMed. Current Medication Information Low-ogestrel (Norgestrel and Ethinyl Estradiol) (March 2007). Available from, as of March 29, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4626
Oral contraceptives should not be used in women who have the following conditions: thrombophlebitis or thromboembolic disorders; a past history of deep vein thrombophlebitis or thromboembolic disorders; cerebral vascular or coronary artery disease; Known or suspected carcinoma of the breast; carcinoma of the endometrium or other known or suspected estrogen-dependent neoplasia; undiagnosed abnormal genital bleeding; cholestatic jaundice of pregnancy or jaundice with prior pill use; hepatic adenomas, carcinomas or benign liver tumors; known or suspected pregnancy
US Natl Inst Health; DailyMed. Current Medication Information Low-ogestrel (Norgestrel and Ethinyl Estradiol) (March 2007). Available from, as of March 29, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4626
The most frequent adverse effect of oral contraceptives is nausea. In addition, nausea has been reported in women using vaginal or transdermal estrogen-progestin contraceptives. The principal risk associated with currently recommended high-dose, postcoital estrogen-progestin combination regimens appears to be moderate to severe adverse GI effects including severe vomiting and nausea, which occur in 12-22 and 30-66%, respectively, of women receiving the short-course regimens and may limit compliance with, and effectiveness of, the regimens. In 2 prospective, randomized studies, nausea and vomiting were less common with a high-dose postcoital progestin-only regimen (0.75 mg levonorgestrel every 12 hours for 2 doses) than with a high-dose estrogen-progestin regimen (100 mcg ethinyl estradiol and 0.5 mg levonorgestrel every 12 hours for 2 doses). Other adverse GI effects include vomiting, abdominal cramps, abdominal pain, bloating, diarrhea, and constipation. Gingivitis and dry socket have also been reported. Changes in appetite and changes in weight also may occur. /Estrogen-Progestin Combination/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3109
For more Drug Warnings (Complete) data for NORGESTREL (52 total), please visit the HSDB record page.
Norgestrel in combination with ethinyl estradiol is indicated for the prevention of pregnancy in women who elect to use this product as a method of contraception.
FDA Label
Contraceptives, Oral, Hormonal
Oral contraceptives which owe their effectiveness to hormonal preparations. (See all compounds classified as Contraceptives, Oral, Hormonal.)
Contraceptives, Oral, Synthetic
Oral contraceptives which owe their effectiveness to synthetic preparations. (See all compounds classified as Contraceptives, Oral, Synthetic.)
Norgestrel is absorbed from the gastrointestinal tract, metabolised by the liver and excreted in the urine and faeces as glucuronide and sulphate conjugates.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Cyclo-Progynova (Last updated March 2011). Available from, as of March 25, 2011: https://www.medicines.org.uk/EMC/medicine/9159/SPC/Cyclo-Progynova+2mg/
(14)C-Norgestrel was administered to seven human subjects and 43% of dose was excreted in the urine within 5 days; the biological half-life of the radioactivity was 24 hr. Enzymic hydrolysis released only 32% of the urinary radioactivity and a further 25% was excreted as sulphate conjugates. The metabolites excreted in the urine were much less polar than those following the administration of the related compounds, norethisterone or lynestrenol. The 3alphaOH,5beta and 3betaOH,5beta isomers of the tetrahydronorgestrel (13beta-ethyl-17alpha-ethynyl-5 beta-gonane-3alpha,17beta-diol) were isolated from urine and identified by mass spectrometry and thin-layer and gas-liquid chromatography. Plasma radioactivity decreased more rapidly than after the administration of norethisterone and lynestrenol. About 2% of the administered dose was converted to acidic compounds. There was no apparent difference in the rate of excretion of radioactivity or in the metabolites after either oral or intravenous administration of norgestrel.
Littleton P et al; Journal of Endocrinology 42: 591-598 (1968)
The binding of different synthetic steroids, used in hormonal contraception, to Sex Hormone Binding Globulin (SHBG) was studied by measuring their ability to displace tritiated testosterone from SHBG in a competitive protein binding system. Only 19-nortestosterone derivates had any significant ability to displace testosterone from SHBG, d-norgestrel (d-Ng) being the strongest displacer. Increasing the SHBG levels in women with previous constant plasma d-Ng levels increased these levels two- to sixfold. It is concluded that SHBG is the main carrier protein for d-Ng. The strong testosterone displacing activity of d-Ng might also explain androgenic side effects observed with d-Ng containig oral contraceptives.
PMID:133117 Victor A et al; J Clin Endocrinol Metab 43 (1): 244-7 (1976)
(14)C-Norgestrel was administered to seven human subjects and 43% of dose was excreted in the urine within 5 days ... Enzymic hydrolysis released only 32% of the urinary radioactivity and a further 25% was excreted as sulphate conjugates. The metabolites excreted in the urine were much less polar than those following the administration of the related compounds, norethisterone or lynestrenol. The 3alphaOH,5beta and 3betaOH,5beta isomers of the tetrahydronorgestrel (13beta-ethyl-17alpha-ethynyl-5 beta-gonane-3alpha,17beta-diol) were isolated from urine and identified by mass spectrometry and thin-layer and gas-liquid chromatography. Plasma radioactivity decreased more rapidly than after the administration of norethisterone and lynestrenol. About 2% of the administered dose was converted to acidic compounds. There was no apparent difference in the rate of excretion of radioactivity or in the metabolites after either oral or intravenous administration of norgestrel.
Littleton P et al; Journal of Endocrinology 42: 591-598 (1968)
The comparative metabolism of dl-, d-, and l-norgestrel was investigated in African Green Monkeys (Cercopithecus aethiops). Total (14)C excretion in urine after a single oral dose of (14)C-dl-norgestrel (1 mg/kg) was significantly higher (51.4 +/- 5.0%) than that observed after administration of the d-enantiomer (37.5 +/- 5.4%) but not the l-enantiomer (44.2 +/- 8.9%). In all cases, the major part of the urinary radioactivity was present in a free fraction (48-62%), while an additional 13-27% was released by beta-glucuronidase preparations. No sulfate conjugates were detected. At least one major (16beta-hydroxylation) and one minor (16alpha-hydroxylation) metabolic pathway were stereoselective, i.e., they are operative with the I-but not the d-enantiomer. Three metabolites, 16beta-hydroxynorgestrel, 16alpha-hydroxynorgestrel, and 16-hydroxytetrahydronorgestrel (believed to be 16beta) were only detected in urine samples obtained from (14)C-dland -l-norgestrel-dosed animals. Following (14)C-d-norgestrel administration, 3alpha, 5beta-tetrahydronorgestrel was found to be the major urinary metabolite. These observations are compared with those reported earlier on the urinary metabolites of dl-norgestrel in women.
Sisenwine S et al; DMD 2 (1): 65-70 (1974)
The in vitro metabolism of stereo-isomers (d, l and the racemic mixture dl) of norgestrel by a microsomal fraction from rabbit liver was investigated. The metabolism of the biologically active l-norgestrel was more rapid than that of d-norgestrel (sic.) which is biologically inactive. This was mainly due to the more ready conversion of l-norgestrel to ring-A reduced metabolites. There was no difference between the two isomers in respect of the amount undergoing hydroxylation; about 40% of each isomer was converted to hydroxylated metabolites after 30 min incubation. However, there were differences between the isomers, l-norgestrel being converted mainly to the 16beta-hydroxysteroid and d-norgestrel to the 16alpha-hydroxysteroid. Similar amounts of both isomers were hydroxylated at C-6. The metabolism of the racemic mixture was intermediate between that of the d and l isomers.
Khana FS, Fotherby K; Journal of Steroid Biochemistry 19 (2): 1169-1172 (1983)
The rates of metabolism of synthetic gestagens derived from 19-nortestosterone by rabbit liver tissue in vitro were compared. Over a period of 1 hr norethisterone was metabolized as rapidly as 19-nortestosterone whereas d-norgestrel and lynestrenol were metabolized at a slightly lower rate. Less than 5% of l-norgestrel was metabolized. In all cases the reaction product was the tetrahydrosteroid. Lynestrenol was metabolised through norethisterone. Skeletal muscle, lung and small intestine also metabolized norethisterone and d-norgestrel but at a slower rate than liver tissue. Small amounts of norethisterone were metabolized by adipose tissue but heart and spleen were inactive. Lynestrenol and l-norgestrel were not metabolized by any of the extra-hepatic tissues studied.
Khana FS, Fotherby K; Journal of Steroid Biochemistry 10 (4): 437-442 (1978)
In vitro studies were conducted on the metabolism of 3 steroids used in OCs (oral contraceptives) by small pieces of human jejunal mucosa. This was done because the gastrointestinal mucosa of humans is known to metabolize a number of drugs. Almost 40% of the ethinyl estradiol, 9.8% of the levonorgestrel, and 7% of the mestranol were metabolized after incubation. All these metabolic responses were significantly different from those in the control groups. Results of the study show that the metabolism of the ethinyl estradiol was related to the weight of the tissue used. These results are consistent with the known marked 1st pass effect of ethinyl estradiol. Norgestrel, known to have little or no 1st pass effect, did not show a high rate of gut metabolism. Under the experimental conditions employed, no Phase 1 metabolism of either ethinyl estradiol or levonorgestrel was apparent.
PMID:6783058 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1401625 Back DJ et al; Br J Clin Pharmacol 11 (3): 275-8 (1981)
(14)C-Norgestrel was administered to seven human subjects and 43% of dose was excreted in the urine within 5 days; the biological half-life of the radioactivity was 24 hr. ...
Littleton P et al; Journal of Endocrinology 42: 591-598 (1968)
Norgestrel (and more specifically the active stereoisomer levonorgestrel) binds to the progesterone and estrogen receptors within the female reproductive tract, the mammary gland, the hypothalamus, and the pituitary. Once bound to the receptor, progestins like levonorgestrel will slow the frequency of release of gonadotropin releasing hormone (GnRH) from the hypothalamus and blunt the pre-ovulatory LH (luteinizing hormone) surge. Loss of the LH surge inhibits ovulation and thereby prevents pregnancy.
Combination oral contraceptives act by suppression of gonadotrophins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cer-vical mucus (which increase the difficulty of sperm entry into the uterus) and the endometrium (which may reduce the likelihood of implantation).
US Natl Inst Health; DailyMed. Current Medication Information Low-ogestrel (Norgestrel and Ethinyl Estradiol) (March 2007). Available from, as of March 29, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4626
Progestins enter target cells by passive diffusion and bind to cytosolic (soluble) receptors that are loosely bound in the nucleus. The steroid receptor complex initiates transcription, resulting in an increase in protein synthesis. /Progestins/
USP. Convention. USPDI - Drug Information for the Health Care Professional. 20th ed. Volume I. Micromedex, Inc. Englewood, CO., 2000. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2568
Progestins are capable of affecting serum concentrations of other hormones, particularly estrogen. Estrogenic effects are modified by the progestins, either by reducing the availability or stability of the hormone receptor complex or by turning off specific hormone-responsive genes by direct interaction with the progestin receptor in the nucleus. In addition, estrogen priming is necessary to increase progestin effects by upregulating the number of progestin receptors and/or increasing progesterone production, causing a negative feedback mechanism that inhibits estrogen receptors. /Progestins/
USP. Convention. USPDI - Drug Information for the Health Care Professional. 20th ed. Volume I. Micromedex, Inc. Englewood, CO., 2000. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2568
There is great concern over the long-term influence of oral contraceptives on the development of breast cancer in women. Estrogens are known to stimulate the growth of human breast cancer cells, and /it/ has previously reported that the 19-norprogestin norethindrone could stimulate the proliferation of MCF-7 human breast cancer cells. /Investigators/ studied the influence of the 19-norprogestins norgestrel and gestodene compared to a 'non' 19-norprogestin medroxyprogesterone acetate (MPA) on MCF-7 cell proliferation. The 19-norprogestins stimulated proliferation at a concentration of 10(-8) M, while MPA could not stimulate proliferation at concentrations as great as 3 x 10(-6) M. The stimulatory activity of the 19-norprogestins could be blocked by the antioestrogen ICI 164,384, but not by the antiprogestin RU486. Transfection studies with the reporter plasmids containing an estrogen response element or progesterone response element (vitERE-CAT, pS2ERE-CAT, and PRE15-CAT) were performed to determine the intracellular action of norgestrel and gestodene. The 19-norprogestins stimulated the vitERE-CAT activity maximally at 10(-6) M, and this stimulation was inhibited by the addition of ICI 164,384. MPA did not stimulate vitERE-CAT activity. A single base pair alteration in the palindromic sequence of vitERE (resulting in the pS2ERE) led to a dramatic decrease in CAT expression by the 19-norprogestins, suggesting that the progestin activity required specific response element base sequencing. PRE15-CAT activity was stimulated by norgestrel, gestodene and MPA at concentrations well below growth stimulatory activity. This stimulation could be blocked by RU486. These studies suggest that the 19-norprogestins norgestrel and gestodene stimulate MCF-7 breast cancer cell growth by activating the estrogen receptor.
PMID:8494728 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1968434 Catherino WH et al; Br J Cancer 67 (5): 945-52 (1993)
ABOUT THIS PAGE
34
PharmaCompass offers a list of Dl Norgestrel API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Dl Norgestrel manufacturer or Dl Norgestrel supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Dl Norgestrel manufacturer or Dl Norgestrel supplier.
PharmaCompass also assists you with knowing the Dl Norgestrel API Price utilized in the formulation of products. Dl Norgestrel API Price is not always fixed or binding as the Dl Norgestrel Price is obtained through a variety of data sources. The Dl Norgestrel Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Dl Norgestrel manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Dl Norgestrel, including repackagers and relabelers. The FDA regulates Dl Norgestrel manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Dl Norgestrel API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Dl Norgestrel supplier is an individual or a company that provides Dl Norgestrel active pharmaceutical ingredient (API) or Dl Norgestrel finished formulations upon request. The Dl Norgestrel suppliers may include Dl Norgestrel API manufacturers, exporters, distributors and traders.
Dl Norgestrel Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Dl Norgestrel GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Dl Norgestrel GMP manufacturer or Dl Norgestrel GMP API supplier for your needs.
A Dl Norgestrel CoA (Certificate of Analysis) is a formal document that attests to Dl Norgestrel's compliance with Dl Norgestrel specifications and serves as a tool for batch-level quality control.
Dl Norgestrel CoA mostly includes findings from lab analyses of a specific batch. For each Dl Norgestrel CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Dl Norgestrel may be tested according to a variety of international standards, such as European Pharmacopoeia (Dl Norgestrel EP), Dl Norgestrel JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Dl Norgestrel USP).
