
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
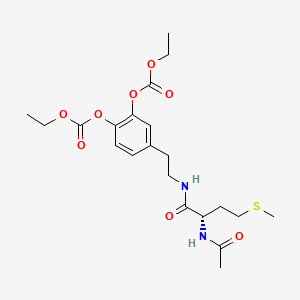

1. Do Carpamine
2. N-(n-acetyl-l-methionyl)-o,o-bis(ethoxycarbonyl)dopamine
3. Ta 870
4. Ta-8704
1. 74639-40-0
2. Tanadopa
3. Docarpamine [inn]
4. Docarpamina
5. Ta-870
6. Rpq57d8s72
7. Dtxsid1057820
8. [4-[2-[[(2s)-2-acetamido-4-methylsulfanylbutanoyl]amino]ethyl]-2-ethoxycarbonyloxyphenyl] Ethyl Carbonate
9. (-)-(s)-2-acetamido-n-(3,4-dihydroxyphenethyl)-4-(methylthio)butyramide Bis(ethyl Carbonate) (ester)
10. Docarpaminum
11. Do Carpamine
12. Docarpaminum [inn-latin]
13. Docarpamina [inn-spanish]
14. (s)-4-(2-(2-acetamido-4-(methylthio)butanamido)ethyl)-1,2-phenylene Diethyl Dicarbonate
15. Tanadopa (tn)
16. Unii-rpq57d8s72
17. Ta-8704
18. N-(n-acetyl-l-methionyl)-o,o-bis(ethoxycarbonyl)dopamine
19. Docarpamine (jan/inn)
20. Docarpamine [mi]
21. Docarpamine [jan]
22. Docarpamine [mart.]
23. Docarpamine [who-dd]
24. Schembl219671
25. Chembl2106351
26. Dtxcid8031609
27. Schembl23356827
28. Chebi:31513
29. Tox21_113707
30. Ncgc00249931-01
31. Carbonic Acid, 4-(2-((2-(acetylamino)-4-(methylthio)-1-oxobutyl)amino)ethyl)-1,2-phenylene Diethyl Ester, (s)-
32. Cas-74639-40-0
33. Ns00127253
34. D01903
35. Q27288234
| Molecular Weight | 470.5 g/mol |
|---|---|
| Molecular Formula | C21H30N2O8S |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 16 |
| Exact Mass | Da |
| Monoisotopic Mass | Da |
| Topological Polar Surface Area | 155 |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 619 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Dopamine Agonists
Drugs that bind to and activate dopamine receptors. (See all compounds classified as Dopamine Agonists.)
ABOUT THIS PAGE
