
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
FDF
0
Canada

0
Australia

0
South Africa

0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
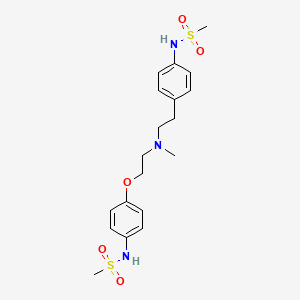

1. 1-(4-methanesulfonamidophenoxy)-2-(n-(4-methanesulfonamidophenethyl)-n-methylamine)ethane
2. 1-mspmpe
3. Tikosyn
4. Uk 68798
5. Uk-68,798
1. 115256-11-6
2. Tikosyn
3. Dofetilida
4. Dofetilidum
5. Dofetilidum [inn-latin]
6. Dofetilida [inn-spanish]
7. Uk 68798
8. Uk-68798
9. Uk-68,798
10. Dofetilide (tikosyn)
11. N-(4-(2-(methyl(2-(4-(methylsulfonamido)phenoxy)ethyl)amino)ethyl)phenyl)methanesulfonamide
12. Beta-((p-methanesulfonamidophenethyl)methylamino)methanesulfono-p-phenetidide
13. Chembl473
14. R4z9x1n2nd
15. N-[4-[2-[2-[4-(methanesulfonamido)phenoxy]ethyl-methylamino]ethyl]phenyl]methanesulfonamide
16. Chebi:4681
17. 1-(4-methanesulphonamidophenoxy)-2-[n-(4-methanesulphonamidophenethyl)-n-methylamino]ethane
18. N-[4-[2-[methyl[2-[4-[(methylsulfonyl)amino]phenoxy]ethyl]amino]ethyl]phenyl]methanesulfonamide
19. Methanesulfonamide, N-[4-[2-[methyl[2-[4-[(methylsulfonyl)amino]phenoxy]ethyl]amino]ethyl]phenyl]-
20. Ncgc00164549-01
21. Uk 68789
22. Dsstox_cid_26433
23. Dsstox_rid_81610
24. Dsstox_gsid_46433
25. Methanesulfonamide, N-(4-(2-(methyl(2-(4-((methylsulfonyl)amino)phenoxy)ethyl)amino)ethyl)phenyl)-
26. Xelide
27. N-(4-{2-[methyl(2-{4-[(methylsulfonyl)amino]phenoxy}ethyl)amino]ethyl}phenyl)methanesulfonamide
28. Smr000466333
29. Tikosyn (tn)
30. Cas-115256-11-6
31. Uk 68,798
32. Unii-r4z9x1n2nd
33. Hsdb 7927
34. 1-(4-methanesulphonamidophenoxy)-2-(n-(4-methanesulphonamidophenethyl)-n-methylamino)ethane
35. Dofetilide [usan:usp:inn:ban]
36. Dofetilide- Bio-x
37. Uk68798
38. Dofetilide [mi]
39. Dofetilide [inn]
40. Dofetilide [jan]
41. Dofetilide [usan]
42. Dofetilide [vandf]
43. Dofetilide [mart.]
44. Dofetilide [usp-rs]
45. Dofetilide [who-dd]
46. Schembl16135
47. Dofetilide (jan/usp/inn)
48. Mls000759442
49. Mls001424185
50. Mls006010154
51. Dofetilide [ema Epar]
52. Gtpl2604
53. Dofetilide [orange Book]
54. Dofetilide, >=98% (hplc)
55. Dtxsid5046433
56. Dofetilide [usp Monograph]
57. Hms2051n14
58. Hms2089f03
59. Hms2232h19
60. Hms3261g03
61. Hms3370b18
62. Hms3393n14
63. Hms3655a17
64. Hms3715h20
65. Zinc596731
66. Act04222
67. Bcp04770
68. Hy-b0232
69. Tox21_112178
70. Tox21_500351
71. Ac-385
72. Bdbm50031720
73. Nsc786034
74. S1658
75. Akos005259921
76. Tox21_112178_1
77. Bcp9000619
78. Ccg-101037
79. Db00204
80. Nc00287
81. Nsc-786034
82. Uk68789
83. Mrf-0000317
84. N-[4-[2-[2-[4-(methanesulfonamido)phenoxy]ethyl-methyl-amino]ethyl]phenyl]methanesulfonamide
85. Ncgc00164549-02
86. Ncgc00261036-01
87. Bd164377
88. Bcp0726000009
89. Db-041252
90. Ft-0631076
91. Sw197667-2
92. C07751
93. D00647
94. H11953
95. Ab00639976-06
96. Ab00639976-08
97. Ab00639976_09
98. 256d116
99. A803396
100. Sr-01000759368
101. J-003271
102. Q3712521
103. Sr-01000759368-4
104. Brd-k86887724-001-05-6
105. Dofetilide, United States Pharmacopeia (usp) Reference Standard
106. .beta.-((p-methanesulfonamidophenethyl)methylamino)methanesulfono-p-phenetidide
107. 1-(4-methansulphonamidophenoxy)-2-[n-(4-methanesulphonamidophenethyl)-n-methylamino]ethane
108. N-[4-[2-[2-(4-methanesulfonamidophenyl)ethyl-methylamino]ethoxy]phenyl]methanesulfonamide
109. (dofetilide) N-[4-(2-{[2-(4-methanesulfonylamino-phenyl)-ethyl]-methylamino}-ethoxy)-phenyl]-methanesulfonamide
110. N-[4-(2-{[2-(4-methanesulfonamidophenoxy)ethyl](methyl)amino}ethyl)phenyl]methanesulfonamide
111. N-[4-(2-{[2-(4-methanesulfonylamino-phenoxy)-ethyl]-methyl-amino}-ethyl)-phenyl]-methanesulfonamide
112. N-[4-(2-{[2-(4-methanesulfonylamino-phenyl)-ethyl]-methyl-amino}-ethoxy)-phenyl]-methanesulfonamide (dofetilide)
113. N-[4-(2-{[2-(4-methanesulfonylamino-phenyl)-ethyl]-methyl-amino}-ethoxy)-phenyl]-methanesulfonamide (uk-68798)
114. N-[4-(2-{[2-(4-methanesulfonylamino-phenyl)-ethyl]-methyl-amino}-ethoxy)-phenyl]-methanesulfonamide Dofetilide
115. N-[4-(2-{2-[4-(methanesulphonamido)phenoxyl]-n-methylethylamino}ethyl)phenyl]-methanesulphonamide
| Molecular Weight | 441.6 g/mol |
|---|---|
| Molecular Formula | C19H27N3O5S2 |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 11 |
| Exact Mass | 441.13921332 g/mol |
| Monoisotopic Mass | 441.13921332 g/mol |
| Topological Polar Surface Area | 122 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 672 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Tikosyn |
| PubMed Health | Dofetilide (By mouth) |
| Drug Classes | Antiarrhythmic, Group III |
| Drug Label | TIKOSYN (dofetilide) is an antiarrhythmic drug with Class III (cardiac action potential duration prolonging) properties. Its empirical formula is C19H27N3O5S2 and it has a molecular weight of 441.6. The structural formula is The chemical name for d... |
| Active Ingredient | Dofetilide |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 0.5mg; 0.25mg; 0.125mg |
| Market Status | Prescription |
| Company | Pfizer |
| 2 of 2 | |
|---|---|
| Drug Name | Tikosyn |
| PubMed Health | Dofetilide (By mouth) |
| Drug Classes | Antiarrhythmic, Group III |
| Drug Label | TIKOSYN (dofetilide) is an antiarrhythmic drug with Class III (cardiac action potential duration prolonging) properties. Its empirical formula is C19H27N3O5S2 and it has a molecular weight of 441.6. The structural formula is The chemical name for d... |
| Active Ingredient | Dofetilide |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 0.5mg; 0.25mg; 0.125mg |
| Market Status | Prescription |
| Company | Pfizer |
Anti-Arrhythmia Agents, Potassium Channel Blockers
National Library of Medicine's Medical Subject Headings online file (MeSH, 2011)
Tikosyn is indicated for the conversion of atrial fibrillation and atrial flutter to normal sinus rhythm. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for TIKOSYN (dofetilide) capsule (April 2010). Available from, as of June 22, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=17543&CFID=75477870&CFTOKEN=ffbdfdb0c7aeadf5-BC7DCA29-F9BA-036F-9F3757A5E6AB046F&jsessionid=ca304d974b4478175376
Tikosyn is indicated for the maintenance of normal sinus rhythm (delay in time to recurrence of atrial fibrillation/atrial flutter (AF/AFl)) in patients with atrial fibrillation/atrial flutter of greater than one week duration who have been converted to normal sinus rhythm. Because Tikosyn can cause life threatening ventricular arrhythmias, it should be reserved for patients in whom atrial fibrillation/atrial flutter is highly symptomatic. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for TIKOSYN (dofetilide) capsule (April 2010). Available from, as of June 22, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=17543&CFID=75477870&CFTOKEN=ffbdfdb0c7aeadf5-BC7DCA29-F9BA-036F-9F3757A5E6AB046F&jsessionid=ca304d974b4478175376
/BOXED WARNING/ To minimize the risk of induced arrhythmia, patients initiated or re-initiated on Tikosyn should be placed for a minimum of 3 days in a facility that can provide calculations of creatinine clearance, continuous electrocardiographic monitoring, and cardiac resuscitation ... . Tikosyn is available only to hospitals and prescribers who have received appropriate Tikosyn dosing and treatment initiation education;
US Natl Inst Health; DailyMed. Current Medication Information for TIKOSYN (dofetilide) capsule (Updated: February 2014). Available from, as of April 23, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=02438044-d6a3-49e9-a1ac-3aad21ef2c8c
Tikosyn (dofetilide) can cause serious ventricular arrhythmias, primarily torsade de pointes (TdP) type ventricular tachycardia, a polymorphic ventricular tachycardia associated with QT interval prolongation. QT interval prolongation is directly related to dofetilide plasma concentration. Factors such as reduced creatinine clearance or certain dofetilide drug interactions will increase dofetilide plasma concentration. The risk of TdP can be reduced by controlling the plasma concentration through adjustment of the initial dofetilide dose according to creatinine clearance and by monitoring the ECG for excessive increases in the QT interval. Treatment with dofetilide must therefore be started only in patients placed for a minimum of three days in a facility that can provide electrocardiographic monitoring and in the presence of personnel trained in the management of serious ventricular arrhythmias. Calculation of the creatinine clearance for all patients must precede administration of the first dose of dofetilide.
US Natl Inst Health; DailyMed. Current Medication Information for TIKOSYN (dofetilide) capsule (April 2010). Available from, as of June 22, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=17543&CFID=75477870&CFTOKEN=ffbdfdb0c7aeadf5-BC7DCA29-F9BA-036F-9F3757A5E6AB046F&jsessionid=ca304d974b4478175376
In patients with mild to moderate renal failure, decreases in dosage based on creatinine clearance are required to minimize the risk of torsades de pointes. The drug should not be used in patients with advanced renal failure or with inhibitors of renal cation transport.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 923
Torsades de pointes occurred in 1-3% of patients in clinical trials where strict exclusion criteria (e.g., hypokalemia) were applied and continuous ECG monitoring was used to detect marked QT prolongation in the hospital. The incidence of this adverse effect during more widespread use of the drug, marketed since 2000, is unknown. Other adverse effects were no more common than with placebo during premarketing clinical trials.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 923
For more Drug Warnings (Complete) data for Dofetilide (16 total), please visit the HSDB record page.
For the maintenance of normal sinus rhythm (delay in time to recurrence of atrial fibrillation/atrial flutter [AF/AFl]) in patients with atrial fibrillation/atrial flutter of greater than one week duration who have been converted to normal sinus rhythm
FDA Label
Tikosyn is a Class III antiarrhythmic agent that is indicated for the following:
- Conversion of persistent atrial fibrillation or atrial flutter to normal sinus rhythm in patients in whom cardioversion by electrical means is not appropriate and in whom the duration of the arrhythmic episode is less than 6 months (see section 5. 1).
- Maintenance of sinus rhythm (after conversion) in patients with persistent atrial fibrillation or atrial flutter. Because TIKOSYN can cause ventricular arrhythmias, it should be reserved for patients in whom atrial fibrillation/atrial flutter is highly symptomatic and in whom other antiarrhythmic therapy is not appropriate.
Dofetilide has not been shown to be effective in patients with paroxysmal atrial arrhythmias (including paroxysmal atrial fibrillation).
Dofetilide is an antiarrhythmic drug with Class III (cardiac action potential duration prolonging) properties and is indicated for the maintenance of normal sinus rhythm. Dofetilide increases the monophasic action potential duration in a predictable, concentration-dependent manner, primarily due to delayed repolarization. At concentrations covering several orders of magnitude, Dofetilide blocks only IKr with no relevant block of the other repolarizing potassium currents (e.g., IKs, IK1). At clinically relevant concentrations, Dofetilide has no effect on sodium channels (associated with Class I effect), adrenergic alpha-receptors, or adrenergic beta-receptors.
Potassium Channel Blockers
A class of drugs that act by inhibition of potassium efflux through cell membranes. Blockade of potassium channels prolongs the duration of ACTION POTENTIALS. They are used as ANTI-ARRHYTHMIA AGENTS and VASODILATOR AGENTS. (See all compounds classified as Potassium Channel Blockers.)
Anti-Arrhythmia Agents
Agents used for the treatment or prevention of cardiac arrhythmias. They may affect the polarization-repolarization phase of the action potential, its excitability or refractoriness, or impulse conduction or membrane responsiveness within cardiac fibers. Anti-arrhythmia agents are often classed into four main groups according to their mechanism of action: sodium channel blockade, beta-adrenergic blockade, repolarization prolongation, or calcium channel blockade. (See all compounds classified as Anti-Arrhythmia Agents.)
C01BD04
C - Cardiovascular system
C01 - Cardiac therapy
C01B - Antiarrhythmics, class i and iii
C01BD - Antiarrhythmics, class iii
C01BD04 - Dofetilide
Absorption
>90%
Volume of Distribution
3 L/kg
Approximately 80% of a single dose of dofetilide is excreted in urine, of which approximately 80% is excreted as unchanged dofetilide with the remaining 20% consisting of inactive or minimally active metabolites. Renal elimination involves both glomerular filtration and active tubular secretion (via the cation transport system, a process that can be inhibited by cimetidine, trimethoprim, prochlorperazine, megestrol and ketoconazole). ...
US Natl Inst Health; DailyMed. Current Medication Information for TIKOSYN (dofetilide) capsule (April 2010). Available from, as of June 22, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=17543&CFID=75477870&CFTOKEN=ffbdfdb0c7aeadf5-BC7DCA29-F9BA-036F-9F3757A5E6AB046F&jsessionid=ca304d974b4478175376
The oral bioavailability of dofetilide is >90%, with maximal plasma concentrations occurring at about 2-3 hours in the fasted state. Oral bioavailability is unaffected by food or antacid. The terminal half life of Tikosyn is approximately 10 hours; steady state plasma concentrations are attained within 2-3 days, with an accumulation index of 1.5 to 2.0. Plasma concentrations are dose proportional. Plasma protein binding of dofetilide is 60-70%, is independent of plasma concentration, and is unaffected by renal impairment. Volume of distribution is 3 L/kg.
US Natl Inst Health; DailyMed. Current Medication Information for TIKOSYN (dofetilide) capsule (April 2010). Available from, as of June 22, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=17543&CFID=75477870&CFTOKEN=ffbdfdb0c7aeadf5-BC7DCA29-F9BA-036F-9F3757A5E6AB046F&jsessionid=ca304d974b4478175376
1. Pharmacokinetics of dofetilide were studied in man, dog, rat and mouse after single IV and oral doses of dofetilide or (14)C-dofetilide. 2. Dofetilide was absorbed completely in all species. Low metabolic clearance in man resulted in complete bioavailability following oral administration. Higher metabolic clearance in rodents, and to a lesser extent dogs, resulted in decreased bioavailability because of first-pass metabolism. 3. Following IV administration, the volume of distribution showed only moderate variation in all species (2.8-6.3 l/kg). High plasma clearance in rodents resulted in short half-life values (mouse 0.32, male rat 0.5 and female rat 1.2 hr), while lower clearance in dog and man gave longer terminal elimination half-lives (4.6 and 7.6 hr respectively). 4. After single IV doses of (14)C-dofetilide, unchanged drug was the major component excreted in urine of all species with several metabolites also present. 5. Metabolites identified in urine from all species were formed by N-oxidation or N-dealkylation of the tertiary nitrogen atom of dofetilide. 6. After oral and IV administration of (14)C-dofetilide to man, parent compound was the only detectable component present in plasma and represented 75% of plasma radioactivity. No single metabolite accounted for greater than 5% of plasma radioactivity.
PMID:1441594 Smith DA et al; Xenobiotica 22 (6): 709-19 (1992)
Hepatic
Dofetilide, a class III antidysrhythmic agent, undergoes both renal and metabolic clearance. Characterization of the metabolism in vitro allows explanation of species differences, whereas identification of the human enzymes involved permits assessment of potential drug interaction. In liver microsomes, the rate of oxidative metabolism of dofetilide is in the order: male rat > female rat > dog > humans, which correlates with the metabolic clearance seen in vivo. In vitro products of oxidative metabolism, formed by N-dealkylation, are the same as those formed in vivo, with the N-desmethyl being the major product. This route of dofetilide metabolism is mediated by cytochrome P450 (CYP). In humans, N-demethylation has a high KM of 657 +/- 116 uM, indicating low affinity for the enzyme's active site. In a number of human liver microsomal preparations, this rate correlated (r = 0.903) with the activity of CYP3A4. There was no correlation with the activities of other isozymes. Specific isozyme inhibitors also indicated the involvement of CYP3A4, with partial inhibition being observed with ketoconazole and troleandeomycin, whereas the activator, alpha-naphthaflavone, caused increased turnover. No inhibition was observed with specific inhibitors or competing substrates for other isozymes. Dofetilide did not significantly inhibit CYP2C9, CYP2D6, or CYP3A4 at concentrations up to 100 microM in vitro. In contrast, amiodarone (IC50, 25 uM) and flecainide (49 microM) inhibited CYP2C9 and quinidine (0.26 uM), and flecainide (0.44 uM) inhibited CYP2D6. Many antidysrhythmic drugs have active, circulating metabolites, complicating the relationship of dose and clinical response. In vitro pharmacology studies allow assessment of the potential contribution to the pharmacological profile by metabolites. Potency of dofetilide and metabolites has been compared for class III (K+ channel blockade) and class I (Na+ channel blockade) antidysrhythmic activities. Three of the metabolites of dofetilide displayed class III activity but at concentrations at least 20-fold higher than dofetilide. Dofetilide N-oxide showed class I activity, but only at high concentration. Neither resting membrane potential or action potential amplitude were affected by any metabolite. This lack of biologically relevant activity is in accord with the close correlation between plasma concentrations of dofetilide and pharmacological response.
PMID:8801060 Walker DK et al; Drug Metab Dispos 24 (4): 447-55 (1996)
Approximately 80% of a single dose of dofetilide is excreted in urine, of which approximately 80% is excreted as unchanged dofetilide with the remaining 20% consisting of inactive or minimally active metabolites. ... In vitro studies with human liver microsomes show that dofetilide can be metabolized by CYP3A4, but it has a low affinity for this isoenzyme. Metabolites are formed by N-dealkylation and N-oxidation. There are no quantifiable metabolites circulating in plasma, but 5 metabolites have been identified in urine
US Natl Inst Health; DailyMed. Current Medication Information for TIKOSYN (dofetilide) capsule (April 2010). Available from, as of June 22, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=17543&CFID=75477870&CFTOKEN=ffbdfdb0c7aeadf5-BC7DCA29-F9BA-036F-9F3757A5E6AB046F&jsessionid=ca304d974b4478175376
10 hours
Following IV administration, ... high plasma clearance in rodents resulted in short half-life values (mouse 0.32, male rat 0.5 and female rat 1.2 hr), while lower clearance in dog and man gave longer terminal elimination half-lives (4.6 and 7.6 hr respectively). ...
PMID:1441594 Smith DA et al; Xenobiotica 22 (6): 709-19 (1992)
The terminal half life of Tikosyn is approximately 10 hours
US Natl Inst Health; DailyMed. Current Medication Information for TIKOSYN (dofetilide) capsule (April 2010). Available from, as of June 22, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=17543&CFID=75477870&CFTOKEN=ffbdfdb0c7aeadf5-BC7DCA29-F9BA-036F-9F3757A5E6AB046F&jsessionid=ca304d974b4478175376
The mechanism of action of Dofetilide is a blockade of the cardiac ion channel carrying the rapid component of the delayed rectifier potassium current, IKr. This inhibition of potassium channels results in a prolongation of action potential duration and the effective refractory period of accessory pathways (both anterograde and retrograde conduction in the accessory pathway).
Rapidly activating delayed rectifier current (IKr) is the key target of class III antiarrhythmic drugs including dofetilide. Due to its complex gating properties, the precise channel state or states that interact with these agents remain poorly defined. We have undertaken a careful analysis of the state dependence of HERG channel block by dofetilide in Xenopus oocytes and Chinese Hamster Ovary (CHO) cells by devising a protocol in which brief sampling pulses were superimposed over a wide range of test potentials. The rate of block onset, maximal steady-state block and IC50 were similar for all test potentials over the activation range, demonstrating that the drug probably interacts with open and/or inactivated but not resting HERG channels with high affinity. Reducing the fraction of inactivated channels at 0 mV by augmenting the external potassium concentration did not alter the sensitivity to dofetilide. In contrast, the S631A and S620T HERG mutations both eliminated inward rectification and reduced dofetilide affinity by approximately 10- and approximately 100-fold respectively. We have also found a novel ultra-slow activation process which occurs in wild type HERG channels at threshold potentials. Overall, ... data imply that dofetilide block occurs equally at all voltages positive to the activation threshold, and that the drug interacts with HERG channels in both the open and inactivated states.
PMID:11907818 Weerapura M et al; Pflugers Arch 443 (4): 520-31 (2002)
Tikosyn (dofetilide) shows Vaughan Williams Class III antiarrhythmic activity. The mechanism of action is blockade of the cardiac ion channel carrying the rapid component of the delayed rectifier potassium current, IKr. At concentrations covering several orders of magnitude, dofetilide blocks only IKr with no relevant block of the other repolarizing potassium currents (e.g., IKs, IK1). At clinically relevant concentrations, dofetilide has no effect on sodium channels (associated with Class I effect), adrenergic alpha-receptors, or adrenergic beta-receptors.
US Natl Inst Health; DailyMed. Current Medication Information for TIKOSYN (dofetilide) capsule (April 2010). Available from, as of June 22, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=17543&CFID=75477870&CFTOKEN=ffbdfdb0c7aeadf5-BC7DCA29-F9BA-036F-9F3757A5E6AB046F&jsessionid=ca304d974b4478175376
The human ether-a-go-go-related gene (HERG) encodes a K+ channel with biophysical properties nearly identical to the rapid component of the cardiac delayed rectifier K+ current (IKr). HERG/IKr channels are a prime target for the pharmacological management of arrhythmias and are selectively blocked by class III antiarrhythmic methanesulfonanilide drugs, such as dofetilide, E4031, and MK-499, at submicromolar concentrations. By contrast, the closely related bovine ether-a-go-go channel (BEAG) is 100-fold less sensitive to dofetilide. To identify the molecular determinants for dofetilide block, we first engineered chimeras between HERG and BEAG and then used site-directed mutagenesis to localize single amino acid residues responsible for block. Using constructs heterologously expressed in Xenopus oocytes, we found that transplantation of the S5-S6 linker from BEAG into HERG removed high-affinity block by dofetilide. A point mutation in the S5-S6 linker region, HERG S620T, abolished high-affinity block and interfered with C-type inactivation. Thus, our results indicate that important determinants of dofetilide binding are localized to the pore region of HERG. Since the loss of high-affinity drug binding was always correlated with a loss of C-type inactivation, it is possible that the changes observed in drug binding are due to indirect allosteric modifications in the structure of the channel protein and not to the direct interaction of dofetilide with the respective mutated site chains. However, the chimeric approach was not able to identify domains outside the S5-S6 linker region of the HERG channel as putative candidates involved in drug binding. Moreover, the reverse mutation BEAG T432S increased the affinity of BEAG K+ channels for dofetilide, whereas C-type inactivation could not be recovered. Thus, the serine in position HERG 620 may participate directly in dofetilide binding; however, an intact C-type inactivation process seems to be crucial for high-affinity drug binding.
PMID:9486667 Ficker E et al; Circ Res 82 (3): 386-9 (1998)

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?