
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
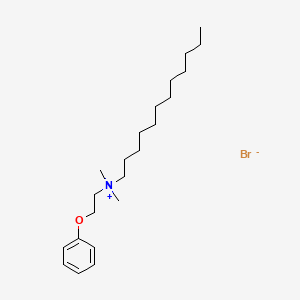

1. Domiphen
2. Domiphen Chloride
3. Domiphen Hydroxide
1. 538-71-6
2. Oradol
3. Domiphen (bromide)
4. Bradasol
5. Fungitex
6. Phenododecinium Bromide
7. 1-dodecanaminium, N,n-dimethyl-n-(2-phenoxyethyl)-, Bromide
8. Nsc-39415
9. N,n-dimethyl-n-(2-phenoxyethyl)dodecan-1-aminium Bromide
10. Dodecyldimethyl(2-phenoxyethyl)ammonium Bromide
11. R4cy19ys7c
12. Ncgc00091010-01
13. Bradonit
14. Bradoral
15. 538-71-6 (br)
16. Bradosol Bromide
17. Fungitex R
18. Dodecyl-dimethyl-(2-phenoxyethyl)azanium;bromide
19. Dsstox_cid_13468
20. Dsstox_rid_79076
21. Dsstox_gsid_33468
22. 1-dodecanaminium, N,n-dimethyl-n-(2-phenoxyethyl)-, Bromide (1:1)
23. Domipheni Bromidum
24. Bromuro De Domifeno
25. Cas-538-71-6
26. Bromure De Domiphene
27. Domipheni Bromidum [inn-latin]
28. Hsdb 7236
29. Bromure De Domiphene [inn-french]
30. Bromuro De Domifeno [inn-spanish]
31. Dodecyldimethyl-2-phenoxyethylammonium Bromide
32. Ncgc00091010-03
33. Einecs 208-702-0
34. Unii-r4cy19ys7c
35. Bradosol
36. Domiphen-bromide
37. Beta-phenoxyethyldimethyldodecylammonium Bromide
38. Ammonium Bromide, Dodecyldimethyl(2-phenoxyethyl)-
39. Mfcd00011769
40. Oradol (tn)
41. Domiphen Bromide [usan:inn:ban:jan]
42. Domiphen Bromide,(s)
43. Domiphen Bromide, 97%
44. Dodecyldimethyl (2-phenoxyethyl) Ammonium Bromide
45. Domiphen Bromide [mi]
46. Schembl117152
47. Ammonium, Dimethyldodecyl(2-phenoxyethyl)-, Bromide
48. Ammonium, Dodecyldimethyl(2-phenoxyethyl)-, Bromide
49. Domiphen Bromide [inn]
50. Domiphen Bromide [jan]
51. Chembl486696
52. Domiphen Bromide [hsdb]
53. Domiphen Bromide [inci]
54. Domiphen Bromide [usan]
55. Domiphen Bromide [vandf]
56. Dtxsid5033468
57. Chebi:31514
58. Domiphen Bromide [mart.]
59. Domiphen Bromide [who-dd]
60. Hms3652l07
61. Domiphen Bromide (jan/usan/inn)
62. Bcp04904
63. Hy-b1467
64. Tox21_111054
65. Tox21_200219
66. S4186
67. Akos003387020
68. Tox21_111054_1
69. Bcp9000621
70. Ccg-268817
71. Cs-5156
72. Ncgc00257773-01
73. Ac-15736
74. As-12368
75. 3-n-fmoc-3-(4-methylphenyl)propionicacid
76. Ft-0625586
77. Sw219200-1
78. Dodecyldimethyl(2-phenoxyethyl)azanium Bromide
79. D01588
80. Sr-01000883678
81. Q4165880
82. Sr-01000883678-1
83. W-105700
84. N,n-dimethyl-n-(2-phenoxyethyl)dodecan-1-aminiumbromide
85. 1-dodecanaminium,n,n-dimethyl-n-(2- Phenoxyethyl)-,bromide
86. Dodecyl-dimethyl-(2-phenoxy-ethyl)-ammonium, Bromide
| Molecular Weight | 414.5 g/mol |
|---|---|
| Molecular Formula | C22H40BrNO |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 15 |
| Exact Mass | 413.22933 g/mol |
| Monoisotopic Mass | 413.22933 g/mol |
| Topological Polar Surface Area | 9.2 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 271 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Anti-infective (topical)
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 601
The efficacy and tolerability of domiphen bromide (domifen bromide), administered at a dosage of 0.5 mg every 4-6 hr in a double-blind placebo controlled study in 31 patients affected by acute infectious dental diseases, are described. After 2 days of treatment with the drug, there was a significant decrease in pain and inflammation. The drug elicited a good response, improved prognosis and reduced the number of days of illness.
PMID:6381338 Scaglione F et al; Int J Clin Pharmacol Res 3 (5): 357-61, (1983)
Domiphen bromide is a quaternary antiseptic with actions and uses similar to those of cationic surfactants.
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 561

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

ABOUT THIS PAGE
38
PharmaCompass offers a list of Domiphen Bromide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Domiphen Bromide manufacturer or Domiphen Bromide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Domiphen Bromide manufacturer or Domiphen Bromide supplier.
PharmaCompass also assists you with knowing the Domiphen Bromide API Price utilized in the formulation of products. Domiphen Bromide API Price is not always fixed or binding as the Domiphen Bromide Price is obtained through a variety of data sources. The Domiphen Bromide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Domiphen Bromide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Domiphen Bromide, including repackagers and relabelers. The FDA regulates Domiphen Bromide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Domiphen Bromide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Domiphen Bromide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Domiphen Bromide supplier is an individual or a company that provides Domiphen Bromide active pharmaceutical ingredient (API) or Domiphen Bromide finished formulations upon request. The Domiphen Bromide suppliers may include Domiphen Bromide API manufacturers, exporters, distributors and traders.
click here to find a list of Domiphen Bromide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Domiphen Bromide DMF (Drug Master File) is a document detailing the whole manufacturing process of Domiphen Bromide active pharmaceutical ingredient (API) in detail. Different forms of Domiphen Bromide DMFs exist exist since differing nations have different regulations, such as Domiphen Bromide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Domiphen Bromide DMF submitted to regulatory agencies in the US is known as a USDMF. Domiphen Bromide USDMF includes data on Domiphen Bromide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Domiphen Bromide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Domiphen Bromide suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Domiphen Bromide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Domiphen Bromide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Domiphen Bromide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Domiphen Bromide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Domiphen Bromide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Domiphen Bromide suppliers with NDC on PharmaCompass.
Domiphen Bromide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Domiphen Bromide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Domiphen Bromide GMP manufacturer or Domiphen Bromide GMP API supplier for your needs.
A Domiphen Bromide CoA (Certificate of Analysis) is a formal document that attests to Domiphen Bromide's compliance with Domiphen Bromide specifications and serves as a tool for batch-level quality control.
Domiphen Bromide CoA mostly includes findings from lab analyses of a specific batch. For each Domiphen Bromide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Domiphen Bromide may be tested according to a variety of international standards, such as European Pharmacopoeia (Domiphen Bromide EP), Domiphen Bromide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Domiphen Bromide USP).
