
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
NDC API
0
VMF
0
Europe

0
Canada

0
Australia

0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
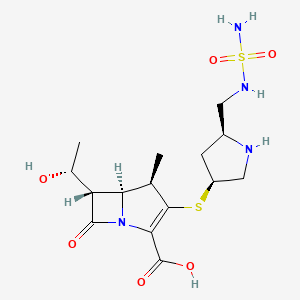

1. 2-(5-sulfamoylaminomethylpyrrolidin-3-ylthio)-6-(1-hydroxyethyl)-1-methylcarbapen-2-em-3-carboxylic Acid
2. S 4661
3. S-4661
4. S4661
1. 148016-81-3
2. Doribax
3. S-4661
4. Finibax
5. Bhv525jobh
6. (4r,5s,6s)-6-[(1r)-1-hydroxyethyl]-4-methyl-7-oxo-3-[(3s,5s)-5-[(sulfamoylamino)methyl]pyrrolidin-3-yl]sulfanyl-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
7. S 4661
8. (4r,5s,6s)-6-((r)-1-hydroxyethyl)-4-methyl-7-oxo-3-(((3s,5s)-5-((sulfamoylamino)methyl)pyrrolidin-3-yl)thio)-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
9. Ncgc00167510-01
10. Drpm
11. Dsstox_cid_26678
12. Dsstox_rid_81814
13. Dsstox_gsid_46678
14. 1-azabicyclo(3.2.0)hept-2-ene-2-carboxylic Acid, 3-(((3s,5s)-5-(((aminosulfonyl)amino)methyl)-3-pyrrolidinyl)thio)-6-((1r)-1-hydroxyethyl)-4-methyl-7-oxo-, (4r,5s,6s)-
15. Doripenem [inn]
16. (+)-(4r,5s,6s)-6-((1r)-1-hydroxyethyl)-4-methyl-7-oxo-3-(((3s,5s)-5-((sulfamoylamino)methyl)-3-pyrrolidinyl)thio)-1-azabicyclo(3.2.0)hept-2-ene-2-carboxylic Acid
17. Cas-148016-81-3
18. Doripenem (usan/inn)
19. Doripenem [usan:inn]
20. Unii-bhv525jobh
21. (4r,5s,6s)-6-[(1r)-1-hydroxyethyl]-4-methyl-7-oxo-3-({(3s,5s)-5-[(sulfamoylamino)methyl]pyrrolidin-3-yl}sulfanyl)-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
22. (4r,5s,6s)-6-[(1r)-1-hydroxyethyl]-4-methyl-7-oxo-3-{[(3s,5s)-5-[(sulfamoylamino)methyl]pyrrolidin-3-yl]sulfanyl}-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
23. Doripenem [mi]
24. Doripenem [usan]
25. (4r,5s,6s)-6-((r)-1-hydroxyethyl)-4-methyl-7-oxo-3-((3s,5s)-5-((sulfamoylamino)methyl)pyrrolidin-3-ylthio)-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
26. Doripenem [vandf]
27. Doripenem [mart.]
28. Doripenem [who-dd]
29. Schembl37471
30. Doripenem [ema Epar]
31. Chembl491571
32. Doripenem [orange Book]
33. Dtxsid2046678
34. Gtpl12183
35. Chebi:135928
36. Bcpp000252
37. Hy-b0187
38. Zinc3922770
39. Tox21_112508
40. Bdbm50088382
41. Mfcd02092739
42. S5531
43. Akos015918362
44. Compound 1m [pmid: 8621362]
45. Tox21_112508_1
46. Am84446
47. Bcp9000623
48. Ccg-268891
49. Cs-2075
50. Db06211
51. Ncgc00167510-02
52. D5249
53. D03895
54. 016d813
55. Q411552
56. Sr-01000872589
57. Sr-01000872589-1
58. (4r,5s,6s)-3-[[(3s,5s)-5-[[(aminosulfonyl)amino]methyl]-3-pyrrolidinyl]thio]-6-[(1r)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
59. (4r,5s,6s)-6-((r)-1-hydroxyethyl)-4-methyl-7-oxo-3-(((3s,5s)-5-((sulfamoylamino)methyl)pyrrolidin-3-yl)thio)-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylicacid
60. 1-azabicyclo(3.2.0)hept-2-ene-2-carboxylic Acid, 3-((5-(((aminosulfonyl)amino)methyl)-3-pyrrolidinyl)thio)-6-(1-hydroxyethyl)-4-methyl-7-oxo-, (4r-(3(3s*,5s*),4alpha,5beta,6beta(r*)))-
61. O6p
| Molecular Weight | 420.5 g/mol |
|---|---|
| Molecular Formula | C15H24N4O6S2 |
| XLogP3 | -3.4 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 7 |
| Exact Mass | 420.11372685 g/mol |
| Monoisotopic Mass | 420.11372685 g/mol |
| Topological Polar Surface Area | 196 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 780 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Doribax |
| PubMed Health | Doripenem (Injection) |
| Drug Classes | Antibiotic |
| Drug Label | DORIBAX, doripenem monohydrate for injection vials contain 500 mg of doripenem on an anhydrous basis, a white to slightly-yellowish off-white sterile crystalline powder. All references to doripenem activity are expressed in terms of the active dori... |
| Active Ingredient | Doripenem |
| Dosage Form | Injectable |
| Route | Iv (infusion) |
| Strength | 500mg/vial; 250mg/vial |
| Market Status | Prescription |
| Company | Shionogi |
| 2 of 2 | |
|---|---|
| Drug Name | Doribax |
| PubMed Health | Doripenem (Injection) |
| Drug Classes | Antibiotic |
| Drug Label | DORIBAX, doripenem monohydrate for injection vials contain 500 mg of doripenem on an anhydrous basis, a white to slightly-yellowish off-white sterile crystalline powder. All references to doripenem activity are expressed in terms of the active dori... |
| Active Ingredient | Doripenem |
| Dosage Form | Injectable |
| Route | Iv (infusion) |
| Strength | 500mg/vial; 250mg/vial |
| Market Status | Prescription |
| Company | Shionogi |
Doripenem is indicated in the treatment of complicated intra-abdominal infections and complicated urinary tract infections, including pyelonephritis, caused by designated susceptible bacteria.
FDA Label
Doribax is indicated for the treatment of the following infections in adults:
- nosocomial pneumonia (including ventilator-associated pneumonia);
- complicated intra-abdominal infections;
- complicated urinary tract infections.
Consideration should be given to official guidance on the appropriate use of antibacterial agents.
Similar to other beta-lactam antimicrobial agents, the time that unbound plasma concentration of doripenem exceeds the MIC (T>MIC) of the infecting organism has been shown to best correlate with efficacy in animal models of infection.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J01DH04
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DH - Carbapenems
J01DH04 - Doripenem
Absorption
Doripenem is administered intravenously as an infusion. There was no accumulation of doripenem following mulitiple infusions of either 500mg or 1g administered every 8 hours for 7-10 days in subjects with normal renal function.
Route of Elimination
Doripenem is primarily eliminated unchanged by the kidneys and undergoes glomerular filtration and active tubular secretion. A mean of 71% and 15% of the dose was recovered in urine as unchanged drug and the ring-opened metabolite, respectively, within 48 hours of 500 mg dose in healthy adults. Following the administration of a single 500 mg dose of radiolabeled doripenem to healthy adults, less than 1% of the total radioactivity was recovered in feces after one week.
Volume of Distribution
The average Vd is 16.8 L (8.09-55.5 L) at steady-state in healthy subjects. Doripenem penetrates into many tissues and fluids, including potential sites of approved indication infections.
Clearance
10.3 L/hour.
Metabolism of doripenem is via dehydropeptidase-I (also called dipeptidase-1) into a microbiologically inactive ring-opened metabolite, doripenem-M1. Doripenem does not appear to be a substrate of the hepatic CYP450 enzymes.
1 hour, in healthy non-elderly adults.
Doripenem is a broad-spectrum carbapenem antibiotic with activity against many gram-positive and gram-negative aerobic bacteria, as well as a variety of anaerobes. Like other beta-lactam antibiotics, doripenem's bactericidal mechanism of action is mostly due to cell death after inhibition of bacterial enzymes called penicillin-bindng proteins (PBPs), which are responsible for peptidoglycan cross-linking during the synthesis of the bacterial cell wall. Carbapenems mainly have high affinity for PBPs 1a, 1b, 2 and 3. Inhibition of each PBP usually results in a different inactivating mechanism. Inhibition of PBPs 1a and 1b results in fast bacterial killing through the formation of spheroplasts, inhibition of PBP 2 results in rod-shaped bacteria to become spherical, and inhibition of PBP 3 results in filamentous-shaped organisms. The PBPs preferentially bound by different carbapenems depend on the organism. In E.coli and P.aeruginosa, doripenem binds to PBP 2, which is involved in the maintenance of cell shape, as well as to PBPs 3 and 4. Doripenem has a 1-beta-methyl side chain, which allows it to be relatively resistant to dehydropeptidase, as well as a trans-alpha-1-hydroxyethyl group at position 6 which provides beta-lactamase resistance. Like other carbapenems, doripenem is different from most beta-lactams due to its stability against hydrolysis by most beta-lactamases, including penicillinases, cephalosporinases, ESBL, and Amp-C producing enterobacteriaceae.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
87
PharmaCompass offers a list of Doripenem API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Doripenem manufacturer or Doripenem supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Doripenem manufacturer or Doripenem supplier.
PharmaCompass also assists you with knowing the Doripenem API Price utilized in the formulation of products. Doripenem API Price is not always fixed or binding as the Doripenem Price is obtained through a variety of data sources. The Doripenem Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Doripenem manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Doripenem, including repackagers and relabelers. The FDA regulates Doripenem manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Doripenem API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Doripenem manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Doripenem supplier is an individual or a company that provides Doripenem active pharmaceutical ingredient (API) or Doripenem finished formulations upon request. The Doripenem suppliers may include Doripenem API manufacturers, exporters, distributors and traders.
click here to find a list of Doripenem suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Doripenem DMF (Drug Master File) is a document detailing the whole manufacturing process of Doripenem active pharmaceutical ingredient (API) in detail. Different forms of Doripenem DMFs exist exist since differing nations have different regulations, such as Doripenem USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Doripenem DMF submitted to regulatory agencies in the US is known as a USDMF. Doripenem USDMF includes data on Doripenem's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Doripenem USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Doripenem suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Doripenem Drug Master File in Korea (Doripenem KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Doripenem. The MFDS reviews the Doripenem KDMF as part of the drug registration process and uses the information provided in the Doripenem KDMF to evaluate the safety and efficacy of the drug.
After submitting a Doripenem KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Doripenem API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Doripenem suppliers with KDMF on PharmaCompass.
Doripenem Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Doripenem GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Doripenem GMP manufacturer or Doripenem GMP API supplier for your needs.
A Doripenem CoA (Certificate of Analysis) is a formal document that attests to Doripenem's compliance with Doripenem specifications and serves as a tool for batch-level quality control.
Doripenem CoA mostly includes findings from lab analyses of a specific batch. For each Doripenem CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Doripenem may be tested according to a variety of international standards, such as European Pharmacopoeia (Doripenem EP), Doripenem JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Doripenem USP).
