
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
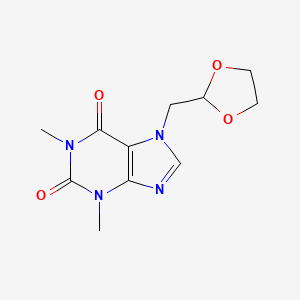

1. 2-(7'-theophyllinemethyl)1,3-dioxolane
2. Doxophylline
3. Tmdo
1. 69975-86-6
2. Doxophylline
3. Ansimar
4. Dioxyfilline
5. Ventax
6. Maxivent
7. Abc 12/3
8. Synasma
9. 7-(1,3-dioxolan-2-ylmethyl)theophylline
10. Mpm23gmo7z
11. Nsc-759645
12. Abc-12/3
13. 1h-purine-2,6-dione, 7-(1,3-dioxolan-2-ylmethyl)-3,7-dihydro-1,3-dimethyl-
14. 7-((1,3-dioxolan-2-yl)methyl)-1,3-dimethyl-1h-purine-2,6(3h,7h)-dione
15. Ncgc00159330-02
16. Dsstox_cid_2968
17. 7-(1,3-dioxolan-2-ylmethyl)-1,3-dimethyl-3,7-dihydro-1h-purine-2,6-dione
18. Dsstox_rid_76810
19. Dsstox_gsid_22968
20. Doxofilina
21. Doxofyllinum
22. Doxofylline [usan:inn]
23. Doxofilina [inn-spanish]
24. Doxofyllinum [inn-latin]
25. 7-((1,3-dioxolan-2-yl)methyl)-1,3-dimethyl-3,7-dihydro-1h-purine-2,6-dione
26. Cas-69975-86-6
27. Sr-01000789760
28. Einecs 274-239-6
29. Unii-mpm23gmo7z
30. Brn 0561195
31. 2-(7'-theophyllinemethyl)-1,3-dioxolane
32. Doxofylline,(s)
33. Maxivent (tn)
34. 2-(7'-teofillinmetil)-1,3-diossolano [italian]
35. Mfcd00865218
36. 2-(7'-teofillinmetil)-1,3-diossolano
37. Doxofylline [mi]
38. Doxofylline [inn]
39. Doxofylline (usan/inn)
40. Doxofylline [usan]
41. Theophylline, 7-(1,3-dioxolan-2-ylmethyl)-
42. 1h-purine-2,6-dione, 3,7-dihydro-7-(1,3-dioxolan-2-ylmethyl)-1,3-dimethyl-
43. 7-(1,3-dioxolon-2-ylmethyl)-1,2,3,6-tetrahydro-1,3-dimethyl-2,6-purindion
44. Doxofylline [mart.]
45. Schembl37963
46. Doxofylline [who-dd]
47. 5-26-14-00120 (beilstein Handbook Reference)
48. Mls001214637
49. Zinc3837
50. Chembl1527608
51. Dtxsid7022968
52. Chebi:94714
53. Doxofylline, >=98% (hplc)
54. 7-(1,3-dioxolan-2-ylmethyl)-1,3-dimethyl-purine-2,6-dione
55. Hms2090e04
56. Hms2877p10
57. Hms3652h03
58. Hms3714m21
59. Hms3885b09
60. Pharmakon1600-01502358
61. Abc-1213
62. Bcp12155
63. Hy-b0004
64. Tox21 111577
65. Tox21_111577
66. Bbl012263
67. Do-309
68. Nsc759645
69. S4164
70. Stk735429
71. 7-(1,3-dioxolan-2-ylmethyl)-3,7-dihydro-1,3-dimethyl-1h-purine-2,6-dione
72. Akos005535592
73. Tox21_111577_1
74. Ac-3492
75. Ccg-213050
76. Cs-8019
77. Db09273
78. Ds-7424
79. Nsc 759645
80. Ncgc00159330-03
81. Ncgc00159330-04
82. Ncgc00159330-10
83. Bd164389
84. Smr000543614
85. D4302
86. Ft-0630792
87. Sw199176-2
88. D03898
89. D90272
90. Ab00828111-06
91. Ab00828111_07
92. Ab00828111_08
93. 975d866
94. A836720
95. L001990
96. Q425887
97. 7-(1,3-dioxolan-2-ylmethyl)-1,3-dimethylxanthine
98. Sr-01000789760-2
99. Sr-01000789760-3
100. 7-(1,3-dioxolan-2-ylmethyl)-1,3-dimethyl-1h-purine-2,6-dione
101. Doxofylline, United States Pharmacopeia (usp) Reference Standard
102. 7-[(1,3-dioxolan-2-yl)methyl]-1,3-dimethyl-2,3,6,7-tetrahydro-1h-purine-2,6-dione
| Molecular Weight | 266.25 g/mol |
|---|---|
| Molecular Formula | C11H14N4O4 |
| XLogP3 | -0.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Exact Mass | 266.10150494 g/mol |
| Monoisotopic Mass | 266.10150494 g/mol |
| Topological Polar Surface Area | 76.9 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 398 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated for the treatment of chronic obstructive pulmonary disease (COPD), bronchial asthma and pulmonary disease with spastic bronchial component.
Doxofylline is a methylxanthine bronchodilator with potent bronchodilator activity comparable to that of theophylline. In animal studies, doxofylline demonstrated to attenuate bronchoconstriction, inflammatory actions and the release of thromboxane A2 (TXA2) when challenged with platelet-activating factor. Doxofylline does not demonstrate direct inhibition of any histone deacetylase (HDAC) enzymes or known PDE enzyme isoforms and did not act as an antagonist at A2 or A2 receptors. The affinity for adenosine A1, A2A and A2B receptors are reported to be all higher than 100 M. It only displays an inhibitory action against PDE2A1 and antagonism at adenosine A(2A) at high concentrations. A study demonstrated that doxofylline interacts with 2-adrenoceptors to induce blood vessel relaxation and airway smooth muscle relaxation. In dog studies, doxofylline decreased airway responsiveness at a dose that did not affect heart rate and respiratory rate.
Antitussive Agents
Agents that suppress cough. They act centrally on the medullary cough center. EXPECTORANTS, also used in the treatment of cough, act locally. (See all compounds classified as Antitussive Agents.)
Bronchodilator Agents
Agents that cause an increase in the expansion of a bronchus or bronchial tubes. (See all compounds classified as Bronchodilator Agents.)
Phosphodiesterase Inhibitors
Compounds which inhibit or antagonize the biosynthesis or actions of phosphodiesterases. (See all compounds classified as Phosphodiesterase Inhibitors.)
R - Respiratory system
R03 - Drugs for obstructive airway diseases
R03D - Other systemic drugs for obstructive airway diseases
R03DA - Xanthines
R03DA11 - Doxofylline
Absorption
After repeated administrations doxofylline reaches the steady-state in about 4 days. Following oral administration of 400 mg doxofylline twice daily for 5 days in adults with chronic bronchitis, the peak plasma concentrations (Cmax) at steady state ranged from 5.78 to 20.76 mcg/mL. The time to reach maximum concentration (Tmax) was 1.19 0.19 hours. The absolute bioavailability of doxofylline in healthy subjects was 63 25%.
Route of Elimination
Less than 4% of an orally administered dose is excreted unchanged in the urine due to extensive hepatic metabolism.
Volume of Distribution
Doxofylline demonstrates a short distribution phase following intravenous administration of 100 mg given in adults with chronic bronchitis. As methylxanthines are distributed to all body compartments, doxofylline may be detected in breast milk and placenta.
Clearance
Following oral administration of 400 mg doxofylline twice daily for 5 days, the total clearance was 555.2 180.6 mL/min.
Doxofylline is thought to undergo hepatic metabolism which accounts for 90% of total drug clearance. -hydroxymethyltheophylline was detected in the serum and urine after oral administration of 400 mg given in healthy subjects. The circulating metabolite was devoid of any significant pharmacological activity.
Following administration of a single intravenous dose of 100 mg over 10 minutes in adults with chronic bronchitis, the elimination half life of doxofylline was 1.83 0.37 hours. Following oral administration of 400 mg twice daily for 5 days in adults with chronic bronchitis, the mean elimination half life was 7.01 0.80 hours.
The main mechanism of action of doxofylline is unclear. One of the mechanisms of action of is thought to arise from the inhibition of phosphodiesterase activity thus increasing the levels of cAMP and promoting smooth muscle relaxation. The interaction of doxofylline with beta-2 adrenoceptors was demonstrated by a study using nonlinear chromatography, frontal analysis and molecular docking. Serine 169 and serine 173 residues in the receptor are thought to be critical binding sites for doxofylline where hydrogen bonds are formed. Via mediating the actions of beta-2 adrenoceptors, doxofylline induces blood vessel relaxation and airway smooth muscle relaxation. There is also evidence that doxofylline may exert anti-inflammatory actions by reducing the pleurisy induced by the inflammatory mediator platelet activating factor (PAF) according to a rat study. It is suggested that doxofylline may play an important role in attenuating leukocyte diapedesis, supported by mouse preclinical studies where doxofylline administration was associated with inhibited leukocyte migration across vascular endothelial cells in vivo and in vitro.Unlike theophylline, doxofylline does not inhibit tumor necrosis factor-induced interleukin (IL)-8 secretion in ASM cells.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
35
PharmaCompass offers a list of Doxofylline API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Doxofylline manufacturer or Doxofylline supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Doxofylline manufacturer or Doxofylline supplier.
PharmaCompass also assists you with knowing the Doxofylline API Price utilized in the formulation of products. Doxofylline API Price is not always fixed or binding as the Doxofylline Price is obtained through a variety of data sources. The Doxofylline Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Doxofylline manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Doxofylline, including repackagers and relabelers. The FDA regulates Doxofylline manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Doxofylline API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Doxofylline manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Doxofylline supplier is an individual or a company that provides Doxofylline active pharmaceutical ingredient (API) or Doxofylline finished formulations upon request. The Doxofylline suppliers may include Doxofylline API manufacturers, exporters, distributors and traders.
click here to find a list of Doxofylline suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Doxofylline DMF (Drug Master File) is a document detailing the whole manufacturing process of Doxofylline active pharmaceutical ingredient (API) in detail. Different forms of Doxofylline DMFs exist exist since differing nations have different regulations, such as Doxofylline USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Doxofylline DMF submitted to regulatory agencies in the US is known as a USDMF. Doxofylline USDMF includes data on Doxofylline's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Doxofylline USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Doxofylline suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Doxofylline Drug Master File in Korea (Doxofylline KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Doxofylline. The MFDS reviews the Doxofylline KDMF as part of the drug registration process and uses the information provided in the Doxofylline KDMF to evaluate the safety and efficacy of the drug.
After submitting a Doxofylline KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Doxofylline API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Doxofylline suppliers with KDMF on PharmaCompass.
A Doxofylline written confirmation (Doxofylline WC) is an official document issued by a regulatory agency to a Doxofylline manufacturer, verifying that the manufacturing facility of a Doxofylline active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Doxofylline APIs or Doxofylline finished pharmaceutical products to another nation, regulatory agencies frequently require a Doxofylline WC (written confirmation) as part of the regulatory process.
click here to find a list of Doxofylline suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Doxofylline as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Doxofylline API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Doxofylline as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Doxofylline and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Doxofylline NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Doxofylline suppliers with NDC on PharmaCompass.
Doxofylline Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Doxofylline GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Doxofylline GMP manufacturer or Doxofylline GMP API supplier for your needs.
A Doxofylline CoA (Certificate of Analysis) is a formal document that attests to Doxofylline's compliance with Doxofylline specifications and serves as a tool for batch-level quality control.
Doxofylline CoA mostly includes findings from lab analyses of a specific batch. For each Doxofylline CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Doxofylline may be tested according to a variety of international standards, such as European Pharmacopoeia (Doxofylline EP), Doxofylline JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Doxofylline USP).
