
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. 63-89-8
2. Dppc
3. Dipalmitoylphosphatidylcholine
4. 1,2-dipalmitoyl-l-lecithin
5. 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine
6. Pc(16:0/16:0)
7. Colfoscerili Palmitas
8. L-dppc
9. Palmitate De Colfosceril
10. Palmitato De Colfoscerilo
11. (r)-2,3-bis(palmitoyloxy)propyl (2-(trimethylammonio)ethyl) Phosphate
12. Dppc, L-
13. Dppc, R-
14. Dipalmitoyl Lecithin, L-
15. 1,2-dipalmitoyl-sn-glycero-3-phosphorylcholine
16. Dipalmitoyl Phosphatidylcholine
17. 1,2-dihexadecanoyl-sn-glycero-3-phosphorylcholine
18. Phosphatidylcholine(32:0)
19. 1,2-dipalmitoyl-3-sn-phosphatidylcholine
20. Dipalmitoyl-phosphatidylcholine
21. 319x2nfw0a
22. Dipalmitoyl Glycerophosphocholine
23. Chebi:72999
24. 129y83
25. Phosphatidylcholine(16:0/16:0)
26. 1-16:0-2-16:0-phosphatidylcholine
27. L-a-dipalmitoyl Lecithin
28. 16:0-16:0-pc
29. Choline Hydroxide, Dihydrogen Phosphate, Inner Salt, Ester With L-1,2-dipalmitin
30. 129-y-83
31. Mfcd00036903
32. Pc 32:0
33. (2-{[(2r)-2,3-bis(hexadecanoyloxy)propyl Phosphonato]oxy}ethyl)trimethylazanium
34. 3,5,9-trioxa-4-phosphapentacosan-1-aminium, 4-hydroxy-n,n,n-trimethyl-10-oxo-7-((1-oxohexadecyl)oxy)-, Hydroxide, Inner Salt, 4-oxide, (r)-
35. L-dipalmitoyl Lecithin
36. 1,2-diacyl-sn-glycero-3-phoshocholine
37. Unii-319x2nfw0a
38. 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine
39. 1,2-dipalmitoyl-phosphatidylcholine
40. Dipalmitoyl-gpc
41. Colfoscerilpalmitat
42. Colfoscerili Palmitas [inn-latin]
43. Colfoscerilpalmitate
44. L-alpha-dppc
45. Einecs 200-567-6
46. L-alpha-dipalmitoyl Phosphatidylcholine
47. Palmitate De Colfosceril [inn-french]
48. Colfosceril Palmitate [usan:inn:ban]
49. L-a-dipalmitoylecithin
50. L-a-dipalmitoyllecithin
51. Palmitato De Colfoscerilo [inn-spanish]
52. L-a-dppc
53. Sn-3-dipalmitoyllecithin
54. Dipalmitoyl-l-a-lecithin
55. L-alpha-dipalmitoylecithin
56. Ncgc00167568-01
57. L-alpha-dipalmitoyllecithin
58. Colfosceril [vandf]
59. Dipalmitoyl-l-alpha-lecithin
60. Dsstox_cid_26720
61. Dsstox_rid_81850
62. Dsstox_gsid_46720
63. Schembl24980
64. L-b,g-dipalmitoyl-a-lecithin
65. Mls000028586
66. Surfaxin Component Dppc
67. 1,2-dipalmitoyl-l-a-lecithin
68. L-1,2-dipalmitoyl-a-lecithin
69. L-a-1,2-dipalmitoyl Lecithin
70. Chembl1200737
71. Dtxsid5046720
72. B,g-dipalmitoyl-l-(a)-lecithin
73. Lucinactant Component Dppc
74. L-a-dipalmitoylphosphatidylcholine
75. L-b,g-dipalmitoyl-alpha-lecithin
76. Phosphatidylcholine 16:0/16:0
77. 1,2-dipalmitoyl-l-alpha-lecithin
78. Colfosceril Palmitate (usan/inn)
79. Colfosceril Palmitate [mi]
80. Hms2231h14
81. Hms3650o14
82. L-1,2-dipalmitoyl-alpha-lecithin
83. L-alpha-1,2-dipalmitoyl Lecithin
84. Colfosceril Palmitate [inn]
85. Dipalmitoyl L-a-phosphatidylcholine
86. Dipalmitoyl-l-a-phosphatidylcholine
87. Amy37583
88. L-b,g-dipalmitoylphosphatidylcholine
89. Colfosceril Palmitate [usan]
90. Dipalmitoyl-sn-3-phosphatidylcholine
91. Tox21_112563
92. B,g-dipalmitoyl-l-phosphatidylcholine
93. Colfosceril Palmitate [vandf]
94. L-1,2-dipalmitoylphosphatidylcholine
95. L-alpha-dipalmitoylphosphatidylcholine
96. Lmgp01010564
97. [(2r)-2,3-di(hexadecanoyloxy)propyl] 2-(trimethylazaniumyl)ethyl Phosphate
98. 1,2-dipalmitoyl-l-phosphatidylcholine
99. Akos016013969
100. Colfosceril Palmitate [who-dd]
101. Dipalmitoyl L-alpha-phosphatidylcholine
102. Dipalmitoyl-l-alpha-phosphatidylcholine
103. 1,2-dipalmitoyl-sn-phosphatidylcholine
104. 1,2-l-a-dipalmitoylphosphatidylcholine
105. B,g-dipalmitoyl L-a-phosphatidylcholine
106. Db09114
107. L-b,g-dipalmitoyl-a-phosphatidylcholine
108. (r)-(4-oxido-10-oxo-7-palmitoyl-3,5,9-trioxa-4-phosphapentacosyl)trimethylammonium 4-oxide
109. 1,2-dipalmitoylglycero-3-phosphocholine
110. Cas-63-89-8
111. 1,2-dipalmitoyl-l-3-phosphatidylcholine
112. 1,2-dipalmitoyl-l-a-phosphatidylcholine
113. 1,2-dipalmitoyl-sn-glycerophosphocholine
114. Dipalmitoyl-l-3-glycerylphosphorylcholine
115. Dihexadecanoyl-sn-glycero-3-phosphocholine
116. Smr000058951
117. 1,2-l-alpha-dipalmitoylphosphatidylcholine
118. Colfosceril Palmitate [orange Book]
119. 1,2-dipalmitoyl-l-alpha-phosphatidylcholine
120. 1,2-dipalmitoyl-sn-3-glycerophosphocholine
121. 1,2-dipalmitoyl-sn-glycerophosphorylcholine
122. B,g-dipalmitoyl L-alpha-phosphatidylcholine
123. Gpc(16:0/16:0)
124. Hy-109506
125. L-b,g-dipalmitoyl-alpha-phosphatidylcholine
126. Cs-0031214
127. D3925
128. 1,2-dipalmitoyl-sn-glycerol-3-phosphocholine
129. 1,2-dipalmitoyl-sn-glyceryl-3-phosphocholine
130. D03585
131. Dipalmitoyl Glycerophosphocholine [inci]
132. H10481
133. Op0201 Component Colfosceril Palmitate
134. 1,2-bis(palmitoyl)-sn-glycero-3-phosphocholine
135. Op-0201 Component Colfosceril Palmitate
136. 1,2-bis(hexadecanoyl)-sn-glycero-3-phosphocholine
137. 1,2-dihexadecanoyl-sn-glycerol-3-phosphorylcholine
138. Q5144764
139. Exosurf Neonatal Component Colfosceril Palmitate
140. 1.2 Dipalmitoyl-sn-glycero-3-phosphatidylcholine
141. Colfosceril Palmitate Component Of Exosurf Neonatal
142. 1,2-dipalmitoyl-sn-glycero-3-phosphocholine, >=99% (tlc)
143. 1.2 Dipalmitoyl-sn-glycero-3-phosphatidylcholine (dppc)
144. (r)-2,3-bis(palmitoyloxy)propyl 2-(trimethylammonio)ethyl Phosphate
145. 1,2-dipalmitoyl-sn-glycero-3-phosphocholine, Semisynthetic, >=99%
146. (2-{[2.3-bis(hexadecanoyloxy)propyl Phosphono]oxy}ethyl)trimethylazanium
147. (2r)-2,3-bis(hexadecanoyloxy)propyl 2-(trimethylammonio)ethyl Phosphate
148. (2r)-2,3-bis(hexadecanoyloxy)propyl 2-(trimethylazaniumyl)ethyl Phosphate
149. 16:0 Pc (dppc), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine, Powder
150. 2-[[(2r)-2,3-di(hexadecanoyloxy)propoxy]-hydroxy-phosphoryl]oxyethyl-trimethyl-ammonium
151. (r)-4-hydroxy-n,n,n-trimethyl-10-oxo-7-[(1-oxohexadecyl)oxy]-3,5,9-trioxa-4-phosphapentacosan-1-aminium 4-oxide Hydroxide Inner Salt
152. (r)-4-hydroxy-n,n,n-trimethyl-10-oxo-7-[(1-oxohexadecyl)oxy]-3,5,9-trioxa-4-phosphapentacosan-1-aminium 4-oxide Inner Salt
1. Dppc
2. Dppc (dipalmitoyl Phosphatidylcholine)
3. (+-)-beta,gamma-dipalmitoyl-alpha-lecithin
4. Dihexadecanoyl Phosphatidylcholine
| Molecular Weight | 734.0 g/mol |
|---|---|
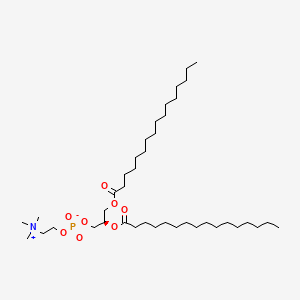
| Molecular Formula | C40H80NO8P |
| XLogP3 | 13.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 40 |
| Exact Mass | 733.56215551 g/mol |
| Monoisotopic Mass | 733.56215551 g/mol |
| Topological Polar Surface Area | 111 Ų |
| Heavy Atom Count | 50 |
| Formal Charge | 0 |
| Complexity | 826 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Colfosceril palmitate is indicated for the treatment of respiratory distress syndrome (RDS) in premature infants. The official label is referred as a intratracheal suspension for prophylactic treatment of infants of less than 1350 grams of birth weight under risk of developing RDS, or in infants with birth weight greater than 1350 grams with pulmonary immaturity, or as rescue treatment of infants that already developed RDS. The central feature of RDS is a surfactant deficiency due to lung immaturity. This lung condition is more frequently presented due to risk factors like prematurity, delayed lung maturation caused by maternal diabetes or male gender, or surfactant dysfuntion due to perinatal asphyxia, pulmonary infection or delivery without labor.
Colfosceril palmitate has shown to significantly reduce the risk of pneumothoraces, pulmonary interstitial emphysema and mortality. Unlike naturals surfactants, colfosceril palmitate reduces the risk of bronchopulmonary dysplasia, intraventricular hemorrhage and patent ductus arteriosus. In clinical placebo-controlled trials, there was a significant reduction in the number of deaths attributed to hyaline membrane disease, the incidence of pulmonary air leaks, oxygen requirements and mean airway pressure. Some reports have indicated a lack of therapeutic effect due to the absence of surfactant protein.
R - Respiratory system
R07 - Other respiratory system products
R07A - Other respiratory system products
R07AA - Lung surfactants
R07AA01 - Colfosceril palmitate
Absorption
The absorption is done directly in the alveolus into the lung tissue. As the lung surfactant is distributed in the bronchi, bronchioles and alveoli, its highest concentration is at the alveolar air-fluid interface where it remains as a monolayer.
Route of Elimination
After 5 days, most of the administered dose (56%) is distributed throughout the body with renal and fecal excretion being the minor elimination pathway representing the 4 and 2% of the eliminated dose respectively. The major route of elimination is by expelled air which accounts for 28% of the administered dose.
Volume of Distribution
Colfosceril palmitate is distributed uniformly to all lobes of the lung, distal airways and alveolar spaces. It will not enter the systemic circulation in healthy lungs, however when the integrity of the tissue is distrupted colfosceril can reach systemic circulation. Even 5 days after administration, there are traces of colfosceril palmitate retained in the body that represented 72% of the administered dose which by then have entered pathways of lipid metabolism to become tissue associated.
Clearance
After 5 days of drug administration, the lung and liver would contain 10% of the administered dose and the elimination via renal excretion accounts only for 8% of the administered dose. This proved a very small renal clearance and confirmed that the major elimination route is by expired air.
Colfosceril palmitate is catabolized and reutilized for further synthesis and secretion in lung tissues.
The half-life of colfosceril palmitate is registered to be in the range of 20-36 hours.
Treatment with colfosceril palmitate aims to reinflate a collapsed area of the lung, improve compliance and reduce intrapulmonary shunting. The actions of colfosceril palmitate are perfomed by replacing the defficient or innefective endogenous lung surfactant and thus, reducing the tension and stabilizing the alveoli from collapsing. Colfosceril palmitate will form a very thin film that will cover the surface of the alveolar cells and therefore it will reduce surface tension.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
65
PharmaCompass offers a list of DPPC API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right DPPC manufacturer or DPPC supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred DPPC manufacturer or DPPC supplier.
PharmaCompass also assists you with knowing the DPPC API Price utilized in the formulation of products. DPPC API Price is not always fixed or binding as the DPPC Price is obtained through a variety of data sources. The DPPC Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A DPPC manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of DPPC, including repackagers and relabelers. The FDA regulates DPPC manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. DPPC API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of DPPC manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A DPPC supplier is an individual or a company that provides DPPC active pharmaceutical ingredient (API) or DPPC finished formulations upon request. The DPPC suppliers may include DPPC API manufacturers, exporters, distributors and traders.
click here to find a list of DPPC suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A DPPC DMF (Drug Master File) is a document detailing the whole manufacturing process of DPPC active pharmaceutical ingredient (API) in detail. Different forms of DPPC DMFs exist exist since differing nations have different regulations, such as DPPC USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A DPPC DMF submitted to regulatory agencies in the US is known as a USDMF. DPPC USDMF includes data on DPPC's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The DPPC USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of DPPC suppliers with USDMF on PharmaCompass.
A DPPC written confirmation (DPPC WC) is an official document issued by a regulatory agency to a DPPC manufacturer, verifying that the manufacturing facility of a DPPC active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting DPPC APIs or DPPC finished pharmaceutical products to another nation, regulatory agencies frequently require a DPPC WC (written confirmation) as part of the regulatory process.
click here to find a list of DPPC suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing DPPC as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for DPPC API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture DPPC as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain DPPC and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a DPPC NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of DPPC suppliers with NDC on PharmaCompass.
DPPC Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of DPPC GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right DPPC GMP manufacturer or DPPC GMP API supplier for your needs.
A DPPC CoA (Certificate of Analysis) is a formal document that attests to DPPC's compliance with DPPC specifications and serves as a tool for batch-level quality control.
DPPC CoA mostly includes findings from lab analyses of a specific batch. For each DPPC CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
DPPC may be tested according to a variety of international standards, such as European Pharmacopoeia (DPPC EP), DPPC JP (Japanese Pharmacopeia) and the US Pharmacopoeia (DPPC USP).
