

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
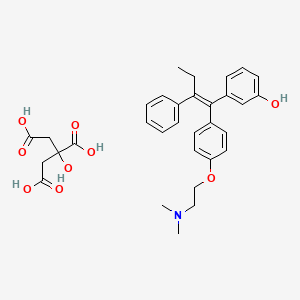

1. 3-hydroxytamoxifen
2. 3-hydroxytamoxifen Citrate
3. Droloxifene
4. Fk 435
5. Fk-435
6. Fk435
7. Meta-hydroxytamoxifen
1. 97752-20-0
2. Droloxifene Citrate [usan]
3. Droloxifenecitrate
4. 6km138nw4b
5. K-060e
6. Droloxifene Citrate (usan)
7. Phenol, 3-(1-(4-(2-(dimethylamino)ethoxy)phenyl)-2-phenyl-1-butenyl)-, (e)-, 2-hydroxy-1,2,3-propanetricarboxylate (1:1) (salt)
8. 3-hydroxytamoxifen Citrate
9. 3-[(e)-1-[4-[2-(dimethylamino)ethoxy]phenyl]-2-phenylbut-1-enyl]phenol;2-hydroxypropane-1,2,3-tricarboxylic Acid
10. Einecs 307-782-5
11. Unii-6km138nw4b
12. Schembl4192
13. (e)-1-(4'-(2-dimethylaminoethoxy)phenyl)-1-(3-hydroxyphenyl)-2-phenylbut-1-ene Citrate
14. (e)-alpha-(p-(2-(dimethylamino)ethoxy)phenyl)-alpha'-ethyl-3-stilbenol Citrate (1:1) (salt)
15. (e)-alpha-(para-(2-(dimethylamino)ethoxy)phenyl)-alpha'-ethyl-3-stilbenol Citrate (iupac)
16. Trans-1-(4-beta-dimethylaminoethoxyphenyl)-1-(3-hydroxyphenyl)-2-phenylbut-1-ene Citrate
17. Chembl2105786
18. Droloxifene Citrate [mi]
19. (e)-(2-(4-(1-(3-hydroxyphenyl)-2-phenylbut-1-enyl)phenoxy)ethyl)dimethylammonium Dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate
20. D03912
21. Q27265061
22. (e)-.alpha.-(p-(2-(dimethylamino)ethoxy)phenyl)-.alpha.'-ethyl-3-stilbenol Citrate (1:1) (salt)
| Molecular Weight | 579.6 g/mol |
|---|---|
| Molecular Formula | C32H37NO9 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 13 |
| Exact Mass | 579.24683176 g/mol |
| Monoisotopic Mass | 579.24683176 g/mol |
| Topological Polar Surface Area | 165 Ų |
| Heavy Atom Count | 42 |
| Formal Charge | 0 |
| Complexity | 728 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Estrogen Antagonists
Compounds which inhibit or antagonize the action or biosynthesis of estrogenic compounds. (See all compounds classified as Estrogen Antagonists.)
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
