
Synopsis
Synopsis
0
KDMF
0
VMF
0
Canada
0
Australia
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
Regulatory FDF Prices
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
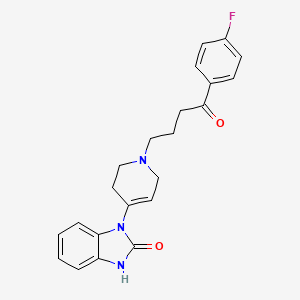

1. Dehidrobenzperidol
2. Dehydrobenzperidol
3. Droleptan
4. Inapsine
1. 548-73-2
2. Droleptan
3. Inapsine
4. Dehydrobenzperidol
5. Dridol
6. Properidol
7. Sintodril
8. Sintosian
9. Inapsin
10. Vetkalm
11. Halkan
12. Deidrobenzperidolo
13. Inopsin
14. Innovar
15. Mcn-jr-4749
16. Inappin
17. Innovan
18. Inoval
19. Dehidrobenzperidol
20. Droperidolum
21. Droperidolo
22. Leptofen
23. Thalamanol
24. Thalamonal
25. Innovar-vet
26. Dihidrobenzperidol
27. R-4749
28. Mcn-jr 4749
29. Component Of Innovar
30. R 4749
31. Nsc 169874
32. 1-(1-(3-(p-fluorobenzoyl)propyl)-1,2,3,6-tetrahydro-4-pyridyl)-2-benzimidazolinone
33. 3-[1-[4-(4-fluorophenyl)-4-oxobutyl]-3,6-dihydro-2h-pyridin-4-yl]-1h-benzimidazol-2-one
34. 1-(1-(4-(p-fluorophenyl)-4-oxobutyl)-1,2,3,6-tetrahydro-4-pyridyl)-2-benzimidazolinone
35. 1-{1-[4-(4-fluorophenyl)-4-oxobutyl]-1,2,3,6-tetrahydropyridin-4-yl}-1,3-dihydro-2h-benzimidazol-2-one
36. Nsc-169874
37. Chembl1108
38. 1-[1-[3-(p-fluorobenzoyl)propyl]-1,2,3,6-tetrahydro-4-pyridyl]-2-benzimidazolinone
39. Mls000028671
40. Chebi:4717
41. O9u0f09d5x
42. 1-(1-(4-(4-fluorophenyl)-4-oxobutyl)-1,2,3,6-tetrahydropyridin-4-yl)-1h-benzo[d]imidazol-2(3h)-one
43. 2h-benzimidazol-2-one, 1-(1-(4-(4-fluorophenyl)-4-oxobutyl)-1,2,3,6-tetrahydro-4-pyridinyl)-1,3-dihydro-
44. 2h-benzimidazol-2-one, 1-[1-[4-(4-fluorophenyl)-4-oxobutyl]-1,2,3,6-tetrahydro-4-pyridinyl]-1,3-dihydro-
45. Dehydrobenzoperidol
46. Nsc169874
47. 1-{1-[4-(4-fluoro-phenyl)-4-oxo-butyl]-1,2,3,6-tetrahydro-pyridin-4-yl}-1,3-dihydro-benzoimidazol-2-one
48. Ncgc00016504-01
49. Cas-548-73-2
50. Droperidolo [dcit]
51. Smr000058855
52. Dsstox_cid_2973
53. Dsstox_rid_76811
54. Dsstox_gsid_22973
55. 1-{1-[4-(4-fluorophenyl)-4-oxobutyl]-1,2,3,6-tetrahydro-4-pyridinyl}-2,3-dihydro-1h-benzo[d]imidazol-2-one
56. Droperidolum [inn-latin]
57. Neurolidol
58. Ina.psi.ne
59. Ina.psi.n
60. Ino.psi.n
61. 1-[1-[4-(p-fluorophenyl)-4-oxobutyl]-1,2,3,6-tetrahydro-4-pyridyl]-2-benzimidazolinone
62. 2-benzimidazolinone, 1-(1-(3-(p-fluorobenzoyl)propyl)-1,2,3,6-tetrahydro-4-pyridyl)-
63. 2-benzimidazolinone, 1-[1-[3-(p-fluorobenzoyl)propyl]-1,2,3,6-tetrahydro-4-pyridyl]-
64. Droleptan (tn)
65. 1-(1-(4-(4-fluorophenyl)-4-oxobutyl)-1,2,3,6-tetrahydro-4-pyridinyl)-1,3-dihydro-2h-benzimidazol-2-one
66. 1-(1-(4-(4-fluorophenyl)-4-oxobutyl)-1,2,3,6-tetrahydropyridin-4-yl)-1,3-dihydro-2h-benzo[d]imidazol-2-one
67. 3-[1-[4-(4-fluorophenyl)-4-oxidanylidene-butyl]-2,3,4,5-tetrahydropyridin-4-yl]-1~{h}-benzimidazol-2-one
68. Ccris 9070
69. Hsdb 3320
70. R4749
71. Sr-05000001546
72. Inapsine (tn)
73. Einecs 208-957-8
74. Brn 0579168
75. Unii-o9u0f09d5x
76. Droperidol Usp
77. 1-(1-[3-(p-fluorobenzoyl)propyl]-1,2,3,6-tetrahydro-4-pyridyl)-2-benzimidazolinone
78. 1-[1-[4-(4-fluorophenyl)-4-oxobutyl]-1,2,3,6-tetrahydro-4-pyridinyl]-1,3-dihydro-2h-benzimidazol-2-one
79. Prestwick_705
80. Mfcd00083290
81. Innovar (salt/mix)
82. Droperidol [usan:usp:inn:ban:jan]
83. Hs-0065
84. Leptanal (salt/mix)
85. Leptofen (salt/mix)
86. Spectrum_001220
87. Thalamonal (salt/mix)
88. Droperidol [mi]
89. Droperidol [inn]
90. Droperidol [jan]
91. Innovar-vet (salt/mix)
92. Opera_id_1751
93. Prestwick0_000360
94. Prestwick1_000360
95. Prestwick2_000360
96. Prestwick3_000360
97. Spectrum2_001386
98. Spectrum3_001426
99. Spectrum4_000407
100. Spectrum5_001305
101. Droperidol [hsdb]
102. Droperidol [usan]
103. Droperidol [vandf]
104. Droperidol [mart.]
105. Droperidol [usp-rs]
106. Droperidol [who-dd]
107. Schembl41426
108. Bspbio_000459
109. Bspbio_003132
110. Kbiogr_000674
111. Kbioss_001700
112. 5-24-02-00388 (beilstein Handbook Reference)
113. Mls000758203
114. Mls001148120
115. Mls001424134
116. Mls002153445
117. Divk1c_000103
118. Spectrum1501002
119. Spbio_001372
120. Spbio_002380
121. Bpbio1_000505
122. Droperidol (jp17/usp/inn)
123. Gtpl7172
124. Droperidol [green Book]
125. Droperidol [orange Book]
126. Dtxsid6022973
127. Hms500f05
128. Kbio1_000103
129. Kbio2_001700
130. Kbio2_004268
131. Kbio2_006836
132. Kbio3_002352
133. Droperidol [ep Monograph]
134. Ninds_000103
135. Droperidol [usp Monograph]
136. Hms1569g21
137. Hms1921b03
138. Hms2051l06
139. Hms2092o16
140. Hms2096g21
141. Hms2232m09
142. Hms3374b05
143. Hms3393l06
144. Hms3652m12
145. Hms3713g21
146. Hms3885n03
147. Pharmakon1600-01501002
148. Innovar Component Droperidol
149. Hy-b1240
150. Tox21_110461
151. Bdbm50017705
152. Ccg-39004
153. Nsc757819
154. S4096
155. Stl453109
156. Zinc19796080
157. Akos015960779
158. Droperidol Component Of Innovar
159. Tox21_110461_1
160. Ac-3537
161. Ccg-101004
162. Cs-4886
163. Db00450
164. Nc00254
165. Nsc-757819
166. 1-1-[3-(p-fluorobenzoyl)propyl]-1,2,3,6-tetrahydro-4-pyridyl-2-benzimidazolinone
167. 3-[1-[4-(4-fluorophenyl)-4-oxo-butyl]-3,6-dihydro-2h-pyridin-4-yl]-1h-benzimidazol-2-one
168. Idi1_000103
169. Ncgc00016504-02
170. Ncgc00016504-03
171. Ncgc00016504-04
172. Ncgc00016504-05
173. Ncgc00016504-06
174. Ncgc00016504-09
175. Ncgc00016504-17
176. Ncgc00094884-01
177. Ncgc00094884-02
178. Sbi-0051628.p002
179. Droperidol 1.0 Mg/ml In Dimethyl Sulfoxide
180. Ft-0655846
181. Sw220019-1
182. D00308
183. F17346
184. Ab00052191_04
185. Ab00052191_05
186. Wln: T56 Bmvnj D3- Dt6n Cutj A3vr Df
187. 548d732
188. A830387
189. L001006
190. Q174259
191. Sr-05000001546-1
192. Sr-05000001546-2
193. Sr-05000001546-3
194. W-105600
195. Brd-k97158071-001-05-8
196. Brd-k97158071-001-08-2
197. Droperidol, British Pharmacopoeia (bp) Reference Standard
198. Droperidol, European Pharmacopoeia (ep) Reference Standard
199. Droperidol, United States Pharmacopeia (usp) Reference Standard
200. 2h-benzimidazol-2-one,2,3,6-tetrahydro-4-pyridinyl]-1,3-dihydro-
201. 1-[1-[3-(p-fluorobenzoyl)propyl]-1,3,6-tetrahydro-4-pyridyl]-2-benzimidazolinone
202. 1-[1-[4-(p-fluorophenyl)-4-oxobutyl]-1,3,6-tetrahydro-4-pyridyl]-2-benzimidazolinone
203. 1-1-[3-(p-fluorobenzoyl)propyl]-1,3,6-tetrahydro-4-pyridyl-2-benzimidazolinone
204. Gamma-[4-(2-oxo-1-benzimidazolinyl)-1,2,3,6-tetrahydro-1-pyridyl]-p-fluorobutyrophenone
205. 1-(4-fluorophenyl)-4-[4-(2-hydroxy-1h-1,3-benzodiazol-1-yl)-1,2,3,6-tetrahydropyridin-1-yl]butan-1-one
206. 1-[1-[4-(4-fluorophenyl)-4-oxo-butyl]-3,6-dihydro-2h-pyridin-4-yl]-3h-benzoimidazol-2-one
207. 1-{1-[4-(4-chloro-phenyl)-4-oxo-butyl]-1,2,3,6-tetrahydro-pyridin-4-yl}-1,3-dihydro-benzoimidazol-2-one(droperidol)
208. 1-{1-[4-(4-fluorophenyl)-4-oxobutyl]-1,2,3,6-tetrahydropyridin-4-yl}-2,3-dihydro-1h-1,3-benzodiazol-2-one
209. 1-{1-[4-(4-fluorophenyl)-4-oxobutyl]-1,2,5,6-tetrahydropyridin-4-yl] -1,3-dihydro-2h-benzimidazol}-2-one
210. 3-[1-[4-(4-fluorophenyl)-4-oxidanylidene-butyl]-3,6-dihydro-2h-pyridin-4-yl]-1h-benzimidazol-2-one
211. Uss
| Molecular Weight | 379.4 g/mol |
|---|---|
| Molecular Formula | C22H22FN3O2 |
| XLogP3 | 3.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | 379.16960512 g/mol |
| Monoisotopic Mass | 379.16960512 g/mol |
| Topological Polar Surface Area | 52.6 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 615 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Droperidol |
| PubMed Health | Droperidol (Injection) |
| Drug Classes | Anesthetic Adjunct, Antiemetic |
| Drug Label | Droperidol Injection, USP, is a sterile, non-pyrogenic, aqueous solution for intravenous or intramuscular use only.Each mL contains:Droperidol.2.5 mgWater for Injection........q.s.p... |
| Active Ingredient | Droperidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2.5mg/ml |
| Market Status | Prescription |
| Company | Hospira; Luitpold |
| 2 of 4 | |
|---|---|
| Drug Name | Inapsine |
| PubMed Health | Droperidol (Injection) |
| Drug Classes | Anesthetic Adjunct, Antiemetic |
| Drug Label | INAPSINE contains droperidol, a neuroleptic (tranquilizer) agent. INAPSINE (droperidol) Injection is available in ampules and vials. Each milliliter contains 2.5 mg of droperidol in an aqueous solution adjusted to pH 3.4 0.4 with lactic acid. Dr... |
| Active Ingredient | Droperidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2.5mg/ml |
| Market Status | Prescription |
| Company | Akorn |
| 3 of 4 | |
|---|---|
| Drug Name | Droperidol |
| PubMed Health | Droperidol (Injection) |
| Drug Classes | Anesthetic Adjunct, Antiemetic |
| Drug Label | Droperidol Injection, USP, is a sterile, non-pyrogenic, aqueous solution for intravenous or intramuscular use only.Each mL contains:Droperidol.2.5 mgWater for Injection........q.s.p... |
| Active Ingredient | Droperidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2.5mg/ml |
| Market Status | Prescription |
| Company | Hospira; Luitpold |
| 4 of 4 | |
|---|---|
| Drug Name | Inapsine |
| PubMed Health | Droperidol (Injection) |
| Drug Classes | Anesthetic Adjunct, Antiemetic |
| Drug Label | INAPSINE contains droperidol, a neuroleptic (tranquilizer) agent. INAPSINE (droperidol) Injection is available in ampules and vials. Each milliliter contains 2.5 mg of droperidol in an aqueous solution adjusted to pH 3.4 0.4 with lactic acid. Dr... |
| Active Ingredient | Droperidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2.5mg/ml |
| Market Status | Prescription |
| Company | Akorn |
Adjuvants, Anesthesia; Antiemetics; Antipsychotic Agents; Dopamine Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Droperidol Injection is indicated to reduce the incidence of nausea and vomiting associated with surgical and diagnostic procedures. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for DROPERIDOL injection, solution (October 2011). Available from, as of June 25, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=147e033d-d997-4ef6-8bb5-a9ba372590b2
Droperidol has been used preoperatively and as an adjunct during induction and maintenance of general anesthesia and as an adjunct to regional anesthesia. /NOT included in US product labeling/
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 2646
Droperidol has been used in combination with an opiate analgesic, such as fentanyl, for neuroleptanalgesia as an anxiolytic and to potentially increase the analgesic effect of the opiate. However, because of the risk of serious adverse effects, the manufacturer no longer recommends these uses. /NOT included in US product labeling/
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 2646
For more Therapeutic Uses (Complete) data for DROPERIDOL (11 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: Cases of QT prolongation and/or torsade de pointes have been reported in patients receiving droperidol at doses at or below recommended doses. Some cases have occurred in patients with no known risk factors for QT prolongation and some cases have been fatal. Due to its potential for serious proarrhythmic effects and death, droperidol should be reserved for use in the treatment of patients who fail to show an acceptable response to other adequate treatments, either because of insufficient effectiveness or the inability to achieve an effective dose due to intolerable adverse effects from those drugs. Cases of QT prolongation and serious arrhythmias (e.g., torsade de pointes) have been reported in patients treated with droperidol. Based on these reports, all patients should undergo a 12-lead ECG prior to administration of droperidol to determine if a prolonged QT interval (i.e., QTc greater than 440 msec for males or 450 msec for females) is present. If there is a prolonged QT interval, droperidol should NOT be administered. For patients in whom the potential benefit of droperidol treatment is felt to outweigh the risks of potentially serious arrhythmias, ECG monitoring should be performed prior to treatment and continued for 2-3 hours after completing treatment to monitor for arrhythmias. Droperidol is contraindicated in patients with known or suspected QT prolongation, including patients with congenital long QT syndrome. Droperidol should be administered with extreme caution to patients who may be at risk for development of prolonged QT syndrome (e.g., congestive heart failure, bradycardia, use of a diuretic, cardiac hypertrophy, hypokalemia, hypomagnesemia, or administration of other drugs known to increase the QT interval). Other risk factors may include age over 65 years, alcohol abuse, and use of agents such as benzodiazepines, volatile anesthetics, and IV opiates. Droperidol should be initiated at a low dose and adjusted upward, with caution, as needed to achieve the desired effect.
US Natl Inst Health; DailyMed. Current Medication Information for DROPERIDOL injection, solution (Updated: November 2013). Available from, as of April 24, 2015: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=147e033d-d997-4ef6-8bb5-a9ba372590b2
Droperidol should be administered with extreme caution to patients who may be at risk for development of prolonged QT syndrome (e.g., congestive heart failure, bradycardia, use of a diuretic, cardiac hypertrophy, hypokalemia, hypomagnesemia, or administration of other drugs known to increase the QT interval). Other risk factors may include age over 65 years, alcohol abuse, and use of agents such as benzodiazepines, volatile anesthetics, and IV opiates. Droperidol should be initiated at a low dose and adjusted upward, with caution, as needed to achieve the desired effect.
US Natl Inst Health; DailyMed. Current Medication Information for DROPERIDOL injection, solution (October 2011). Available from, as of June 25, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=147e033d-d997-4ef6-8bb5-a9ba372590b2
Droperidol is contraindicated in patients with known or suspected QT prolongation (i.e., QTc interval greater than 440 msec for males or 450 msec for females). This would include patients with congenital long QT syndrome.
US Natl Inst Health; DailyMed. Current Medication Information for DROPERIDOL injection, solution (October 2011). Available from, as of June 25, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=147e033d-d997-4ef6-8bb5-a9ba372590b2
Droperidol is contraindicated in patients with known hypersensitivity to the drug.
US Natl Inst Health; DailyMed. Current Medication Information for DROPERIDOL injection, solution (October 2011). Available from, as of June 25, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=147e033d-d997-4ef6-8bb5-a9ba372590b2
For more Drug Warnings (Complete) data for DROPERIDOL (26 total), please visit the HSDB record page.
Droperidol is used to produce tranquilization and to reduce the incidence of nausea and vomiting in surgical and diagnostic procedures.
Droperidol produces marked tranquilization and sedation. It allays apprehension and provides a state of mental detachment and indifference while maintaining a state of reflex alertness. Droperidol produces an antiemetic effect as evidenced by the antagonism of apomorphine in dogs. It lowers the incidence of nausea and vomiting during surgical procedures and provides antiemetic protection in the postoperative period. Droperidol potentiates other CNS depressants. It produces mild alpha-adrenergic blockade, peripheral vascular dilatation and reduction of the pressor effect of epinephrine. It can produce hypotension and decreased peripheral vascular resistance and may decrease pulmonary arterial pressure (particularly if it is abnormally high). It may reduce the incidence of epinephrine-induced arrhythmias, but it does not prevent other cardiac arrhythmias.
Adjuvants, Anesthesia
Agents that are administered in association with anesthetics to increase effectiveness, improve delivery, or decrease required dosage. (See all compounds classified as Adjuvants, Anesthesia.)
Antiemetics
Drugs used to prevent NAUSEA or VOMITING. (See all compounds classified as Antiemetics.)
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
Dopamine D2 Receptor Antagonists
Compounds and drugs that bind to and inhibit or block the activation of DOPAMINE D2 RECEPTORS. (See all compounds classified as Dopamine D2 Receptor Antagonists.)
N05AD08
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N05 - Psycholeptics
N05A - Antipsychotics
N05AD - Butyrophenone derivatives
N05AD08 - Droperidol
Absorption
Completely absorbed following intramuscular administration.
Following im or iv administration, the onset of pharmacologic action of droperidol occurs within 3-10 minutes, but peak pharmacologic effects may not be apparent until 30 minutes. The sedative and tranquilizing effects of droperidol generally persist for 2-4 hours following im or iv administration of a single dose; alteration of consciousness may persist for up to 12 hours.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 2648
Droperidol reportedly crosses the blood-brain barrier and is distributed into the CSF.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 2648
Droperidol drug reportedly crosses the placenta, but data are limited.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 2648
It is not known if droperidol is distributed into milk.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 2648
For more Absorption, Distribution and Excretion (Complete) data for DROPERIDOL (7 total), please visit the HSDB record page.
Extensively metabolized.
Although the exact metabolic fate of droperidol is not clearly established, the drug is metabolized in the liver. The butyrophenone moiety of droperidol is metabolized to p-fluorophenylacetic acid, which is then conjugated with glycine. The nitrogenous moiety of droperidol appears to be metabolized to benzimidazolone and p-hydroxypiperidine.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 2648
Biphasic distribution. The rapid distribution phase is 1.4 ± 0.5 minutes and the slower distribution phase is 14.3 ± 6.5 minutes. Elimination half-life in adults is 134 ± 13 minutes and may be increased in geriatric patients. In children, it is 101.5 ± 26.4 minutes.
...Droperidol is rapidly absorbed after im injections, and plasma-level profiles of unchanged drug obey 2-compartment model kinetics. Plasma t1/2 is about 130 min...
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 122
The exact mechanism of action is unknown, however, droperidol causes a CNS depression at subcortical levels of the brain, midbrain, and brainstem reticular formation. It may antagonize the actions of glutamic acid within the extrapyramidal system. It may also inhibit cathecolamine receptors and the reuptake of neurotransmiters and has strong central antidopaminergic action and weak central anticholinergic action. It can also produce ganglionic blockade and reduced affective response. The main actions seem to stem from its potent Dopamine(2) receptor antagonism with minor antagonistic effects on alpha-1 adrenergic receptors as well.
Within the CNS, droperidol acts principally at subcortical levels and exhibits a strong sedative effect. The drug also inhibits sympathetic postganglionic alpha-adrenergic receptor binding sites; however, therapeutic dosages of droperidol do not appear to completely block these receptors. Droperidol has antiemetic activity, but does not appear to exhibit analgesic activity.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 2648
Because of its inhibitory effects on alpha-adrenergic receptor binding sites, droperidol attenuates the cardiovascular response to sympathomimetic amines. Droperidol also produces direct peripheral vasodilation, which alone or in conjunction with its alpha-adrenergic blocking activity may cause hypotension and decreased peripheral vascular resistance. Droperidol may decrease pulmonary arterial pressure (particularly in patients with preexisting elevations in pulmonary arterial pressure) and reduce the incidence of epinephrine-induced arrhythmias; however, droperidol's activity against other arrhythmias has not been observed.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 2648
Droperidol produces the inhibition of K+ channels in cardiac myocytes. However, the effects of droperidol on K+ channels have not been studied in blood vessels. Therefore, /investigators/ designed the present study to determine whether droperidol modulates the activity of adenosine triphosphate (ATP)-sensitive K+ channels in vascular smooth muscle cells. Rat aortic rings without endothelium were suspended or used for isometric force and membrane potential recordings, respectively. Vasorelaxation and hyperpolarization induced by levcromakalim (10(-8) to 10(-5) M or 10(-5) M, respectively) were completely abolished by the ATP-sensitive K+ channel antagonist glibenclamide (10(-5) M). Droperidol (10(-7) M) and an alpha-adrenergic receptor antagonist phentolamine (3 x 10(-9) M) caused a similar vasodilator effect (approximately 20% of vasorelaxation compared with maximal vasorelaxation induced by papaverine 3 x 10(-4) M, whereas glibenclamide did not alter vasorelaxation induced by droperidol. Droperidol (3 x 10(-8) M to 10(-7) M) augmented vasorelaxation and hyperpolarization produced by levcromakalim, whereas phentolamine (3 x 10(-9) M) did not alter this vasorelaxation. Glibenclamide (10(-5) M) abolished the vasodilating and hyperpolarizing effects of levcromakalim in the aorta treated with droperidol (10(-7) M). These results suggest that droperidol augments vasodilator activity via ATP-sensitive K+ channels. However, it is unlikely that this augmentation is mediated by the inhibition of alpha-adrenergic receptors in vascular smooth muscles.
PMID:16492829 Kinoshita H et al; Anesth Analg 102 (3): 786-91 (2006)
... An inhibitory dopaminergic receptor has been described that modulates catecholamine release from adrenal medulla. It has also been reported that low doses of droperidol increase arterial pressure in some patients with pheochromocytoma. The authors investigated whether an effect of droperidol on such a receptor could be one of the mechanisms involved in the hypertensive response. Isolated cat adrenal glands were perfused with Krebs-bicarbonate solution, and the catecholamine release was measured in the effluent. Then, the glands were stimulated by activation of the nicotinic receptor (nicotine, 5 uM), and the effect of low and high doses of droperidol and/or apomorphine on the catecholamine secretory responses evoked by nicotine was investigated. Low concentrations of droperidol (0.05 uM) (a dopaminergic antagonist) markedly increased the secretory response induced by nicotine whereas higher concentrations (50 uM) decreased it. Apomorphine (1 uM) (a dopaminergic agonist) inhibits the catecholamine release produced by nicotine, and this inhibitory effect was completely reversed by the lowest concentration of droperidol but not by the highest. In fact, the high concentration of droperidol further inhibited the catecholamine release induced by nicotine. The results suggest that the hypertensive responses evoked by low doses of droperidol in some patients with pheochromocytoma could be due to the inactivation of a dopaminergic inhibitory system present in the adrenal medulla that, under physiologic conditions, limits the amount of catecholamines released by the gland. Such as an inhibitory mechanism could operate in an exaggerated manner in patients with pheochromocytoma.
PMID:3777477 Montiel C et al; Anesthesiology 65 (5): 474-9 (1996)
For more Mechanism of Action (Complete) data for DROPERIDOL (6 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
59
PharmaCompass offers a list of Droperidol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Droperidol manufacturer or Droperidol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Droperidol manufacturer or Droperidol supplier.
PharmaCompass also assists you with knowing the Droperidol API Price utilized in the formulation of products. Droperidol API Price is not always fixed or binding as the Droperidol Price is obtained through a variety of data sources. The Droperidol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Droperidol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Droperidol, including repackagers and relabelers. The FDA regulates Droperidol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Droperidol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Droperidol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Droperidol supplier is an individual or a company that provides Droperidol active pharmaceutical ingredient (API) or Droperidol finished formulations upon request. The Droperidol suppliers may include Droperidol API manufacturers, exporters, distributors and traders.
click here to find a list of Droperidol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Droperidol DMF (Drug Master File) is a document detailing the whole manufacturing process of Droperidol active pharmaceutical ingredient (API) in detail. Different forms of Droperidol DMFs exist exist since differing nations have different regulations, such as Droperidol USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Droperidol DMF submitted to regulatory agencies in the US is known as a USDMF. Droperidol USDMF includes data on Droperidol's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Droperidol USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Droperidol suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Droperidol Drug Master File in Japan (Droperidol JDMF) empowers Droperidol API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Droperidol JDMF during the approval evaluation for pharmaceutical products. At the time of Droperidol JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Droperidol suppliers with JDMF on PharmaCompass.
A Droperidol CEP of the European Pharmacopoeia monograph is often referred to as a Droperidol Certificate of Suitability (COS). The purpose of a Droperidol CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Droperidol EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Droperidol to their clients by showing that a Droperidol CEP has been issued for it. The manufacturer submits a Droperidol CEP (COS) as part of the market authorization procedure, and it takes on the role of a Droperidol CEP holder for the record. Additionally, the data presented in the Droperidol CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Droperidol DMF.
A Droperidol CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Droperidol CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Droperidol suppliers with CEP (COS) on PharmaCompass.
A Droperidol written confirmation (Droperidol WC) is an official document issued by a regulatory agency to a Droperidol manufacturer, verifying that the manufacturing facility of a Droperidol active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Droperidol APIs or Droperidol finished pharmaceutical products to another nation, regulatory agencies frequently require a Droperidol WC (written confirmation) as part of the regulatory process.
click here to find a list of Droperidol suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Droperidol as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Droperidol API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Droperidol as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Droperidol and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Droperidol NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Droperidol suppliers with NDC on PharmaCompass.
Droperidol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Droperidol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Droperidol GMP manufacturer or Droperidol GMP API supplier for your needs.
A Droperidol CoA (Certificate of Analysis) is a formal document that attests to Droperidol's compliance with Droperidol specifications and serves as a tool for batch-level quality control.
Droperidol CoA mostly includes findings from lab analyses of a specific batch. For each Droperidol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Droperidol may be tested according to a variety of international standards, such as European Pharmacopoeia (Droperidol EP), Droperidol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Droperidol USP).

