
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
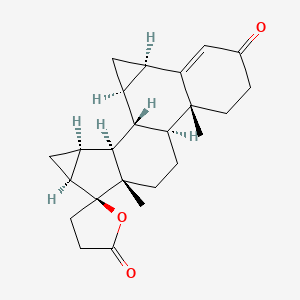

1. 1,2-dihydro-spirorenone
2. 1,2-dihydrospirorenone
3. 6beta,7beta,15beta,16beta-dimethylen-3-oxo-17alpha-pregn-4-en-21,17-carbolacton
4. Dihydrospirorenone
5. Zk 30595
6. Zk30595
1. 67392-87-4
2. Dihydrospirorenone
3. Dehydrospirorenone
4. Drospirenona
5. 1,2-dihydrospirorenone
6. Drsp
7. Drospirenonum
8. Drospirenonum [inn-latin]
9. Drospirenona [inn-spanish]
10. Zk 30595
11. Slynd
12. Zk-30595
13. Chebi:50838
14. N295j34a25
15. Nsc-760103
16. (6r,7r,8r,9s,10r,13s,14s,15s,16s,17s)-1,3',4',6,6a,7,8,9,10,11,12,13,14,15,15a,16-hexadecahydro-10,13-dimethylspiro-(17h-dicyclopropa(6,7:15,16)cyclopenta(a)phenanthrene-17,2'(5'h)-furan)-3,5'(2h)-dione
17. Drospirenone [inn]
18. Dsstox_cid_26465
19. Dsstox_rid_81639
20. Dsstox_gsid_46465
21. 6beta,7beta;15beta,16beta-dimethylene-3-oxo-17alpha-pregn-4-ene-21,17-carbolactone
22. (1r,2r,4r,10r,11s,14s,15s,16s,18s,19s)-10,14-dimethylspiro[hexacyclo[9.8.0.0^{2,4}.0^{5,10}.0^{14,19}.0^{16,18}]nonadecane-15,2'-oxolan]-5-ene-5',7-dione
23. Cas-67392-87-4
24. Zk30595
25. Ccris 6523
26. Einecs 266-679-2
27. Brn 4765500
28. Unii-n295j34a25
29. Hsdb 7896
30. Ncgc00164590-01
31. Drospirenone [usan:usp:inn:ban]
32. Mfcd00867350
33. Sh-470
34. Drospirenone- Bio-x
35. Slynd (tn)
36. 1, 2-dihydrospirorenone
37. Drospirenone [mi]
38. Drospirenone [jan]
39. Drospirenone [usan]
40. 6-beta,7-beta;15-beta,16-beta-dimethylene-3-oxo-17-alpha-pregn-4-ene-21,17-carbolactone
41. Chembl1509
42. Drospirenone [vandf]
43. Drospirenone [mart.]
44. 17-hydroxy-6beta,7beta:15beta,16beta-dimethylene-3-oxo-17alpha-pregn-4-ene-21-carboxylic Acid, Gamma-lactone
45. Spiro(17h-dicyclopropa(6,7:15,16)cyclopenta(a)phenanthrene-17,2'(5'h)-furan)-3,5'(2h)-dione, 1,3',4',6,7,8,9,10,11,12,13,14,15,16,20,21-hexadecahydro-10,13-dimethyl-, (6r-(6alpha,7alpha,8beta,9alpha,10beta,13beta,14alpha,15alpha,16alpha,17beta))-
46. Drospirenone [usp-rs]
47. Drospirenone [who-dd]
48. Schembl153316
49. Drospirenone (jan/usp/inn)
50. Gtpl2874
51. Dtxsid7046465
52. Yaz Component Drospirenone
53. Drospirenone, >=98% (hplc)
54. Bcpp000250
55. Bdbm150275
56. Drospirenone [orange Book]
57. Beyaz Component Drospirenone
58. Drospirenone [ep Monograph]
59. Bcp27035
60. Drospirenone For Peak Identification
61. Ex-a3153
62. Hy-b0111
63. Yasmin Component Drospirenone
64. Zinc3927200
65. Drospirenone [usp Monograph]
66. Drospirenone Component Of Yaz
67. Tox21_112216
68. Angeliq Component Drospirenone
69. S1377
70. Safyral Component Drospirenone
71. Akos015895237
72. Drospirenone Component Of Beyaz
73. Tox21_112216_1
74. Bcp9000628
75. Ccg-268245
76. Cs-1863
77. Db01395
78. Drospirenone Component Of Yasmin
79. Nsc 760103
80. Zk 3059
81. 6beta,7beta,15beta,16beta-dimethylene-3-oxo-17alpha-pregn-4-ene-21,17 Carbolactone
82. Drospirenone Component Of Angeliq
83. Drospirenone Component Of Safyral
84. Ncgc00164590-02
85. Ncgc00164590-04
86. Nextstellis Component Drospirenone
87. As-13025
88. Bd166530
89. Us8987239, A
90. Drospirenone Component Of Nextstellis
91. D4209
92. D03917
93. Ab01274783-01
94. Ab01274783_02
95. 392d874
96. A846434
97. Ar-270/43507886
98. Q419646
99. Sr-01000942231
100. Q-101411
101. Sr-01000942231-1
102. Brd-k04394237-001-06-0
103. Drospirenone, European Pharmacopoeia (ep) Reference Standard
104. Drospirenone, United States Pharmacopeia (usp) Reference Standard
105. Drospirenone For Peak Identification, European Pharmacopoeia (ep) Reference Standard
106. (1r,2r,4r,10r,11s,14s,15s,16s,18s,19s)-10,14-dimethylspiro[hexacyclo[9.8.0.02,4.05,10.014,19.016,18]nonadec-5-ene-15,5'-oxolane]-2',7-dione
107. 17-hydroxy-6.beta.,7.beta.:15.beta.,16.beta.-dimethylene-3-oxo-17.alpha.-pregn-4-ene-21-carboxylic Acid, .gamma.-lactone
108. 3-oxo-6alpha,7alpha,15alpha,16alpha-tetrahydro-7'h,16'h-dicyclopropa[6,7;15,16]-17alpha-pregn-4-ene-21,17-carbolactone
109. 5a,7a-dimethyl-1,1a,3,3',4,4',5,5a,5b,6,7,7a,8a,9,9a,9b,9c,9d-octaadecahydrospiro(cyclopropa[4,5]cyclopenta[1,2-a]cyclopropa[l]phenanthren-8,5'-furan)-2',3-dione
110. 6beta,7beta:15beta,16beta -dimethylene-3-oxo-17alpha-pregn-4-ene-21,17-carbolactone, Dihydrospirorenone
111. Spiro[8h-cyclopropa[4,5]cyclopenta[1,2-a]cyclopropa[l]phenanthrene-8,2'(5'h)-furan]-3,5'(4h)-dione, 1,1a,3',4',5,5a,5b,6,7,7a,8a,9,9a,9b,9c,9d-hexadecahydro-5a,7a-dimethyl-, (1ar,5ar,5bs,7as,8s,8as,9a R,9bs,9cr,9dr)-
| Molecular Weight | 366.5 g/mol |
|---|---|
| Molecular Formula | C24H30O3 |
| XLogP3 | 3.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 366.21949481 g/mol |
| Monoisotopic Mass | 366.21949481 g/mol |
| Topological Polar Surface Area | 43.4 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 828 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 10 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Androstenes; Progesterone Congeners; Aldosterone Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Gianvi is indicated for the prevention of pregnancy in women who elect to use an oral contraceptive. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information GIANVI (drospirenone and ethinyl estradiol) kit (November 2010). Available from, as of February 22, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=31973
Gianvi is indicated for the treatment of symptoms of premenstrual dysphoric disorder (PMDD) in women who choose to use an oral contraceptive as their method of contraception. The effectiveness of Gianvi for PMDD when used for more than three menstrual cycles has not been evaluated. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information GIANVI (drospirenone and ethinyl estradiol) kit (November 2010). Available from, as of February 22, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=31973
Gianvi has not been evaluated for the treatment of premenstrual syndrome (PMS). /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information GIANVI (drospirenone and ethinyl estradiol) kit (November 2010). Available from, as of February 22, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=31973
Gianvi is indicated for the treatment of moderate acne vulgaris in women at least 14 years of age, who have no known contraindications to oral contraceptive therapy and have achieved menarche. Gianvi should be used for the treatment of acne only if the patient desires an oral contraceptive for birth control. /included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information GIANVI (drospirenone and ethinyl estradiol) kit (November 2010). Available from, as of February 22, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=31973
/BOXED WARNING/ WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS. Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptives (COC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, COCs should not be used by women who are over 35 years of age and smoke
US Natl Inst Health; DailyMed. Current Medication Information GIANVI (drospirenone and ethinyl estradiol) kit (Updated: April 2012). Available from, as of April 23, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=723d0427-cf3f-4ad7-8b93-cb1250f345f6
Cigarette smoking increases the risk of serious cardiovascular side effects from oral contraceptive use. This risk increases with age and with heavy smoking (15 or more cigarettes per day) and is quite marked in women over 35 years of age. Women who use oral contraceptives should be strongly advised not to smoke.
US Natl Inst Health; DailyMed. Current Medication Information GIANVI (drospirenone and ethinyl estradiol) kit (November 2010). Available from, as of February 22, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=31973
Gianvi contains ... the progestin drospirenone that has antimineralocorticoid activity, including the potential for hyperkalemia in high-risk patients, comparable to a ... dose of spironolactone. Gianvi should not be used in patients with conditions that predispose to hyperkalemia (ie renal insufficiency, hepatic dysfunction and adrenal insufficiency). Women receiving daily, long-term treatment for chronic conditions or diseases with medications that may increase serum potassium should have their serum potassium level checked during the first treatment cycle. Medications that may increase serum potassium include ACE inhibitors, angiotensin - II receptor antagonists, potassium-sparing diuretics, potassium supplementation, heparin, aldosterone antagonists, and NSAIDS.
US Natl Inst Health; DailyMed. Current Medication Information GIANVI (drospirenone and ethinyl estradiol) kit (November 2010). Available from, as of February 22, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=31973
Use of oral contraceptives is associated with an increased risk of several serious conditions including thromboembolism, stroke, myocardial infarction, liver tumor, gallbladder disease, visual disturbances, fetal abnormalities, and hypertension. Cigarette smoking increases the risk of serious adverse cardiovascular effects during oral contraceptive use. This risk increases with age and with heavy smoking (15 or more cigarettes daily) and is markedly greater in women older than 35 years of age. Women who are receiving estrogen-progestin contraceptives should be strongly advised not to smoke. Women older than 35 years of age who smoke, and women with ischemic heart disease or a history of this disease, should not use estrogen-progestin contraceptives. Estrogen-progestin contraceptives should be used with caution in women with cardiovascular disease risk factors. /Estrogen-Progestin Combination/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3112
For more Drug Warnings (Complete) data for Drospirenone (39 total), please visit the HSDB record page.
Drospirenone, in combination with ethinyl estradiol or estetrol, is indicated as an oral contraceptive for the prevention of pregnancy. In addition to its use for contraceptive effects, this combination is used to treat moderate acne vulgaris and the symptoms of premenstrual dysphoric disorder. The drug has approved indications for combination with estrogens for the treatment of menopause-associated symptoms, such as vasomotor symptoms and vulvovaginal atrophy. Drospirenone combined with estrogen may also may aid in the prevention of osteoporosis in women who have been post-menopausal for at least a year and are not candidates for other therapies. It can sometimes be found in preparations containing estrogen and folic acid for folic acid replenishment during oral contraception. When used for the treatment of acne vulgaris, drospirenone-containing contraceptives should only be used in women 14 years of age who have experienced menarche, desire oral contraception, and do not have any contraindications to oral contraceptives. Off-label uses for this drug include the treatment of menstrual irregularities, dysmenorrhea, hirsutism, and endometriosis.
FDA Label
Prevention of pregnancy
Drospirenone inhibits the maturation of follicles and inhibits ovulation, preventing pregnancy. It has antiandrogen effects, improving acne and hirsutism. When combined with ethinyl estradiol, it has been shown to have favorable effects on the plasma lipid profile. Due to its similarity to naturally occurring progesterone, drospirenone is thought to be associated with a lower incidence of progesterone contraceptive related adverse effects, such as breast tenderness and mood swings. **A note on venous thromboembolism risk and antimineralcorticoid effects** As with other oral contraceptives, the risk of venous thromboembolism and cardiovascular events may be increased when drospirenone is taken. The risk is especially higher in smokers and women aged 35 and older. Women taking this drug should be advised not to smoke. In addition, drospirenone, due to its antimineralcorticoid effects, may increase the risk of hyperkalemia. Patients at high risk for hyperkalemia should not be administered this drug. Consult the official prescribing information for detailed and updated information on the cardiovascular and other risks associated with drospirenone use.
Mineralocorticoid Receptor Antagonists
Drugs that bind to and block the activation of MINERALOCORTICOID RECEPTORS by MINERALOCORTICOIDS such as ALDOSTERONE. (See all compounds classified as Mineralocorticoid Receptor Antagonists.)
G03FA17
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
G - Genito urinary system and sex hormones
G03 - Sex hormones and modulators of the genital system
G03A - Hormonal contraceptives for systemic use
G03AC - Progestogens
G03AC10 - Drospirenone
Absorption
The absolute bioavailability of drospirenone is approximately 76% due to first-pass effects. The maximum plasma concentration of drospirenone occurs within 1 to 2 hours after oral administration and is estimated to range between 60 and 87 ng/mL. A European prescribing monograph for the combination product of estradiol and drospirenone indicates that drospirenone is both completely and rapidly absorbed. It reports a Cmax of 21.9 ng/ml, achieved approximately 1-hour post-administration. The absolute bioavailability is reported to range between 76 to 85%.
Route of Elimination
Various metabolites of drospirenone are measured in the urine and feces. Drospirenone elimination from the body is almost after 10 days post-administration when negligible amounts of drospirenone are found unchanged in both the urine and feces. Between 38% to 47% of the metabolites are identified as glucuronide and sulfate conjugates in the urine. In the feces, approximately 17% to 20% of identifiable metabolites are found to be excreted as glucuronides and sulfates.
Volume of Distribution
The volume of distribution of drospirenone is estimated to be 4 L/kg, according to the FDA label for Yaz. Prescribing information from a combination of estradiol and drospirenone estimates the volume of distribution to range from 3.7- 4.2 L/kg.
Clearance
Drospirenone is rapidly cleared, typically within 2-3 days of administration of the last active tablet. The rate of clearance of drospirenone calculated in the serum ranges from 1.2-1.5 ml/min/kg, however, this value can vary by up to 25% according to the patient.
The absolute bioavailability of drospirenone (DRSP) from a single entity tablet is about 76%. Serum concentrations of DRSP and EE reached peak levels within 1-2 hours after administration of Gianvi.
US Natl Inst Health; DailyMed. Current Medication Information GIANVI (drospirenone and ethinyl estradiol) kit (November 2010). Available from, as of February 22, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=31973
The pharmacokinetics of DRSP are dose proportional following single doses ranging from 1-10 mg. Following daily dosing of Gianvi, steady state DRSP concentrations were observed after 8 days. There was about 2 to 3 fold accumulation in serum Cmax and AUC (0-24hr) values of DRSP following multiple dose administration of Gianvi.
US Natl Inst Health; DailyMed. Current Medication Information GIANVI (drospirenone and ethinyl estradiol) kit (November 2010). Available from, as of February 22, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=31973
The rate of absorption of DRSP and EE following single administration of a formulation similar to Gianvi was slower under fed (high fat meal) conditions with the serum Cmax being reduced about 40% for both components. The extent of absorption of DRSP, however, remained unchanged.
US Natl Inst Health; DailyMed. Current Medication Information GIANVI (drospirenone and ethinyl estradiol) kit (November 2010). Available from, as of February 22, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=31973
DRSP and EE serum levels decline in two phases. The apparent volume of distribution of DRSP is approximately 4 L/kg and that of EE is reported to be approximately 4-5 L/kg.
US Natl Inst Health; DailyMed. Current Medication Information GIANVI (drospirenone and ethinyl estradiol) kit (November 2010). Available from, as of February 22, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=31973
For more Absorption, Distribution and Excretion (Complete) data for Drospirenone (10 total), please visit the HSDB record page.
Drospirenone is heavily metabolized. The two major inactive metabolites identified are the acid form of drospirenone produced by the opening of its lactone ring, known as M11, and the 4,5-dihydro-drospirenone-3-sulfate (M14). Drospirenone also undergoes oxidative metabolism via the hepatic cytochrome enzyme CYP3A4.
The two main metabolites of DRSP found in human plasma were identified to be the acid form of DRSP generated by opening of the lactone ring and the 4,5-dihydrodrospirenone-3-sulfate. These metabolites were shown not to be pharmacologically active. In in vitro studies with human liver microsomes, DRSP was metabolized only to a minor extent mainly by Cytochrome P450 3A4 (CYP3A4).
US Natl Inst Health; DailyMed. Current Medication Information GIANVI (drospirenone and ethinyl estradiol) kit (November 2010). Available from, as of February 22, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=31973
The serum half-life of drospirenone is estimated to be 30 hours. The half-life of drospirenone metabolite excretion in the urine and feces is approximately 40 hours.
DRSP serum levels are characterized by a terminal disposition phase half-life of approximately 30 hours after both single and multiple dose regimens.
US Natl Inst Health; DailyMed. Current Medication Information GIANVI (drospirenone and ethinyl estradiol) kit (November 2010). Available from, as of February 22, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=31973
Drospirenone and ethinyl estradiol in combination suppress the release of follicle stimulating hormone (FSH) and luteinizing hormone (LH), preventing ovulation. Other changes induced by this drug which may aid in the prevention of pregnancy include alterations in cervical mucus consistency, hindering sperm movement, and lowering the chance of embryo implantation. Drospirenone is an analog of the diuretic spironolactone, which exerts anti-mineralocorticoid activity, blocking aldosterone receptors, which increases sodium and water excretion. Studies in animals have demonstrated that drospirenone administration leads to antiandrogenic activity. This activity helps to oppose the effects of naturally occurring androgens, inhibiting the binding of dihydrotestosterone (DHT) to its receptor, and preventing androgen synthesis in the ovaries, helping to treat acne and hirsutism. Drospirenone may also decrease the level of edema in sebaceous follicle during the second half of the menstrual cycle, when acne often appears.
Combination oral contraceptives (COCs) act by suppression of gonadotropins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cervical mucus (which increases the difficulty of sperm entry into the uterus) and the endometrium (which reduces the likelihood of implantation).
US Natl Inst Health; DailyMed. Current Medication Information GIANVI (drospirenone and ethinyl estradiol) kit (November 2010). Available from, as of February 22, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=31973
Drospirenone is a spironolactone analogue with antimineralocorticoid activity. Preclinical studies in animals and in vitro have shown that drospirenone has no androgenic, estrogenic, glucocorticoid, or antiglucocorticoid activity. Preclinical studies in animals have also shown that drospirenone has antiandrogenic activity.
US Natl Inst Health; DailyMed. Current Medication Information GIANVI (drospirenone and ethinyl estradiol) kit (November 2010). Available from, as of February 22, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=31973
Acne vulgaris is a skin condition with a multifactorial etiology including androgen stimulation of sebum production. While the combination of ethinyl estradiol and drospirenone increases sex hormone binding globulin (SHBG) and decreases free testosterone, the relationship between these changes and a decrease in the severity of facial acne in otherwise healthy women with this skin condition has not been established. The impact of the antiandrogenic activity of drospirenone on acne is not known.
US Natl Inst Health; DailyMed. Current Medication Information GIANVI (drospirenone and ethinyl estradiol) kit (November 2010). Available from, as of February 22, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=31973
.... The pharmacological properties of drospirenone were investigated in vitro by receptor binding and transactivation experiments and in vivo in appropriate animal models. In qualitative agreement with progesterone, the compound binds strongly to the progesterone and the mineralocorticoid receptor and with lower affinity to androgen and glucocorticoid receptors. There is no detectable binding to the estrogen receptor. Steroid hormone agonistic and antagonistic activities of progesterone and drospirenone were compared in transactivation experiments. Individual steroid hormone receptors were artificially expressed together with a reporter gene in appropriate cell lines. Both hormones were unable to induce any androgen receptor-mediated agonistic activity. Rather, both progesterone and drospirenone distinctly antagonized androgen-stimulated transcriptional activation. Likewise, both compounds only very weakly activated the mineralocorticoid receptor but showed potent aldosterone antagonistic activity. Drospirenone did not induce glucocorticoid receptor-driven transactivation. Progesterone was a weak agonist in this respect. Drospirenone exerts potent progestogenic and antigonadotropic activity which was studied in various animal species. It efficiently promotes the maintenance of pregnancy in ovariectomized rats, inhibits ovulation in rats and mice and stimulates endometrial transformation in the rabbit. Furthermore, drospirenone shows potent antigonadotropic, i.e., testosterone-lowering activity in male cynomolgus monkeys. The progestogenic potency of drospirenone was found to be in the range of that of norethisterone acetate. The majority of clinically used progestogens are androgenic. Drospirenone, like progesterone, has no androgenic but rather an antiandrogenic effect. This property was demonstrated in castrated, testosterone propionate substituted male rats by a dose-dependent inhibition of accessory sex organ growth (seminal vesicles, prostate). In this model, the potency of drospirenone was about a third that of cyproterone acetate. Drospirenone, like progesterone, shows antimineralocorticoid activity, which causes moderately increased sodium and water excretion. This is an outstanding characteristic which has not been described for any other synthetic progestogen before. Drospirenone is eight to ten times more effective in this respect than spironolactone. The natriuretic effect was demonstrable for at least three weeks upon daily treatment of rats with a dose of 10 mg/animal. Drospirenone is devoid of any estrogenic, glucocorticoid or antiglucocorticoid activity. In summary, drospirenone, like progesterone, combines potent progestogenic with antimineralocorticoid and antiandrogenic activity in a similar dose range.
PMID:7625729 Muhn P et al; Ann N Y Acad Sci. 761: 311-35 (1995)
For more Mechanism of Action (Complete) data for Drospirenone (9 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
75
PharmaCompass offers a list of Drospirenone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Drospirenone manufacturer or Drospirenone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Drospirenone manufacturer or Drospirenone supplier.
PharmaCompass also assists you with knowing the Drospirenone API Price utilized in the formulation of products. Drospirenone API Price is not always fixed or binding as the Drospirenone Price is obtained through a variety of data sources. The Drospirenone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Drospirenone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Drospirenone, including repackagers and relabelers. The FDA regulates Drospirenone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Drospirenone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Drospirenone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Drospirenone supplier is an individual or a company that provides Drospirenone active pharmaceutical ingredient (API) or Drospirenone finished formulations upon request. The Drospirenone suppliers may include Drospirenone API manufacturers, exporters, distributors and traders.
click here to find a list of Drospirenone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Drospirenone DMF (Drug Master File) is a document detailing the whole manufacturing process of Drospirenone active pharmaceutical ingredient (API) in detail. Different forms of Drospirenone DMFs exist exist since differing nations have different regulations, such as Drospirenone USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Drospirenone DMF submitted to regulatory agencies in the US is known as a USDMF. Drospirenone USDMF includes data on Drospirenone's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Drospirenone USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Drospirenone suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Drospirenone Drug Master File in Japan (Drospirenone JDMF) empowers Drospirenone API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Drospirenone JDMF during the approval evaluation for pharmaceutical products. At the time of Drospirenone JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Drospirenone suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Drospirenone Drug Master File in Korea (Drospirenone KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Drospirenone. The MFDS reviews the Drospirenone KDMF as part of the drug registration process and uses the information provided in the Drospirenone KDMF to evaluate the safety and efficacy of the drug.
After submitting a Drospirenone KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Drospirenone API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Drospirenone suppliers with KDMF on PharmaCompass.
A Drospirenone CEP of the European Pharmacopoeia monograph is often referred to as a Drospirenone Certificate of Suitability (COS). The purpose of a Drospirenone CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Drospirenone EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Drospirenone to their clients by showing that a Drospirenone CEP has been issued for it. The manufacturer submits a Drospirenone CEP (COS) as part of the market authorization procedure, and it takes on the role of a Drospirenone CEP holder for the record. Additionally, the data presented in the Drospirenone CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Drospirenone DMF.
A Drospirenone CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Drospirenone CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Drospirenone suppliers with CEP (COS) on PharmaCompass.
A Drospirenone written confirmation (Drospirenone WC) is an official document issued by a regulatory agency to a Drospirenone manufacturer, verifying that the manufacturing facility of a Drospirenone active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Drospirenone APIs or Drospirenone finished pharmaceutical products to another nation, regulatory agencies frequently require a Drospirenone WC (written confirmation) as part of the regulatory process.
click here to find a list of Drospirenone suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Drospirenone as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Drospirenone API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Drospirenone as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Drospirenone and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Drospirenone NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Drospirenone suppliers with NDC on PharmaCompass.
Drospirenone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Drospirenone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Drospirenone GMP manufacturer or Drospirenone GMP API supplier for your needs.
A Drospirenone CoA (Certificate of Analysis) is a formal document that attests to Drospirenone's compliance with Drospirenone specifications and serves as a tool for batch-level quality control.
Drospirenone CoA mostly includes findings from lab analyses of a specific batch. For each Drospirenone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Drospirenone may be tested according to a variety of international standards, such as European Pharmacopoeia (Drospirenone EP), Drospirenone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Drospirenone USP).
