


API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
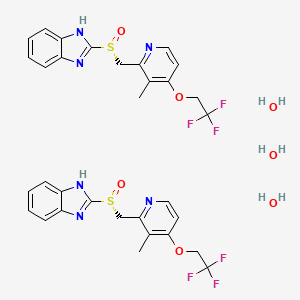

1. 2-((r)-((3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl)methyl)sulfinyl)-1h-benzimidazole
2. Dexilant
3. Dexlansoprazole
4. Lansoprazole, R Isomer
5. Lansoprazole, R-isomer
6. R Lansoprazole
7. R-isomer Lansoprazole
8. R-lansoprazole
9. T 168390
10. T-168390
11. T168390
12. Tak 390
13. Tak 390mr
14. Tak-390
15. Tak-390mr
16. Tak390
17. Tak390mr
1. Hs2s9vk3nh
2. 313640-86-7
3. Unii-hs2s9vk3nh
4. Dtxsid50185290
5. Q27280070
6. 1h-benzimidazole, 2-((r)-((3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl)methyl)sulfinyl)-, Hydrate (2:3)
| Molecular Weight | 792.8 g/mol |
|---|---|
| Molecular Formula | C32H34F6N6O7S2 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 19 |
| Rotatable Bond Count | 10 |
| Exact Mass | 792.18345876 g/mol |
| Monoisotopic Mass | 792.18345876 g/mol |
| Topological Polar Surface Area | 177 Ų |
| Heavy Atom Count | 53 |
| Formal Charge | 0 |
| Complexity | 480 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 5 |
Anti-Ulcer Agents
Various agents with different action mechanisms used to treat or ameliorate PEPTIC ULCER or irritation of the gastrointestinal tract. This has included ANTIBIOTICS to treat HELICOBACTER INFECTIONS; HISTAMINE H2 ANTAGONISTS to reduce GASTRIC ACID secretion; and ANTACIDS for symptomatic relief. (See all compounds classified as Anti-Ulcer Agents.)
Proton Pump Inhibitors
Compounds that inhibit H(+)-K(+)-EXCHANGING ATPASE. They are used as ANTI-ULCER AGENTS and sometimes in place of HISTAMINE H2 ANTAGONISTS for GASTROESOPHAGEAL REFLUX. (See all compounds classified as Proton Pump Inhibitors.)


