
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
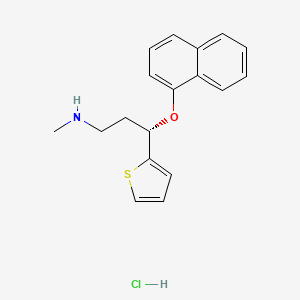

1. Cymbalta
2. Duloxetine
3. Duloxetine Ethanedioate (1:1), (+-)-isomer - T353987
4. Duloxetine Hcl
5. Duloxetine, (+)-isomer
6. Hcl, Duloxetine
7. Hydrochloride, Duloxetine
8. Ly 227942
9. Ly 248686
10. Ly-227942
11. Ly-248686
12. Ly227942
13. Ly248686
14. N-methyl-3-(1-naphthalenyloxy)-2-thiophenepropanamine
15. N-methyl-3-(1-naphthalenyloxy)-3-(2-thiophene)propanamide
1. 136434-34-9
2. Duloxetine Hcl
3. Cymbalta
4. (s)-duloxetine Hydrochloride
5. Ariclaim
6. Xeristar
7. (s)-n-methyl-3-(naphthalen-1-yloxy)-3-(thiophen-2-yl)propan-1-amine Hydrochloride
8. Duloxetine Hydrochloride [usan]
9. Duloxetine Mylan
10. Duloxetine (hydrochloride)
11. Ly248686 Hcl
12. Ly-248686 Hcl
13. Nsc-759112
14. Cpd000469136
15. Duloxetine (as Hydrochloride)
16. Chebi:31526
17. (3s)-n-methyl-3-naphthalen-1-yloxy-3-thiophen-2-ylpropan-1-amine;hydrochloride
18. 9044sc542w
19. Yentreve (tn)
20. (+)-(s)-n-methyl-gamma-(1-naphthyloxy)-2-thiophenepropylamine Hydrochloride
21. Dsstox_cid_26443
22. Dsstox_rid_81618
23. Dsstox_gsid_46443
24. Duloxetine Boehringer Ingelheim
25. Methyl[3-(naphthalen-1-yloxy)-3-(thiophen-2-yl)propyl]amine Hydrochloride
26. (+)-(s)-n-methyl-.gamma.-(1-naphthyloxy)-2-thiophenepropylamine Hydrochloride
27. (s)-n-methyl-3-(naphthalen-1-yloxy)-3-(thiophen-2-yl)propan-1-amine Hcl
28. Smr000469136
29. Dulane 20
30. Cas-136434-34-9
31. Ncgc00164559-01
32. C18h19nos.hcl
33. Unii-9044sc542w
34. Ly 248686 Hcl
35. Cymbalta (tn)
36. Duloxetina Cloridrato
37. Duloxetine Hydrochlorise
38. Duloxetine Lilly
39. Cloridrato De Duloxetina
40. Clorhidrato De Duloxetina
41. Chlorhydrate De Duloxetine
42. Duloxetine Hcl (cymbalta)
43. Mls001401452
44. Mls006010054
45. (s)-duloxetine (hydrochloride)
46. Duloxetine Hydrochloride- Bio-x
47. Chembl1200328
48. Dtxsid9046443
49. Duloxetine Hydrochloride Solution
50. Duloxetine For System Suitability
51. Hy-b0161a
52. Pharmakon1600-01505387
53. Ly-248686 Hydrochloride
54. Amy12420
55. Duloxetine Hydrochloride (jan/usp)
56. Tox21_112188
57. Ac-924
58. Duloxetine Hydrochloride [mi]
59. Mfcd06407958
60. Nsc744012
61. Nsc759112
62. S2084
63. Duloxetine Hydrochloride [jan]
64. (3s)-n-methyl-3-(1-naphthyloxy)-3-(2-thienyl)propan-1-amine Hydrochloride
65. Akos016340453
66. Tox21_112188_1
67. Ccg-101106
68. Cs-1993
69. Ks-1168
70. Nc00356
71. Nsc 759112
72. Nsc-744012
73. (3s)-n-methyl-3-naphthalen-1-yloxy-3-thiophen-2-ylpropan-1-amine Hydrochloride
74. 2-thiophenepropanamine, N-methyl-gamma-(1-naphthalenyloxy)-, Hydrochloride, (gammas)-
75. 2-thiophenepropanamine, N-methyl-gamma-(1-naphthalenyloxy)-, Hydrochloride, (s)-
76. Duloxetine Hydrochloride [mart.]
77. Duloxetine Hydrochloride [usp-rs]
78. Duloxetine Hydrochloride [who-dd]
79. Ncgc00164559-03
80. (s)-(+)-duloxetine Hydrochloride
81. Bd165546
82. Ly-264453
83. D-170
84. D4223
85. Sw197393-3
86. Duloxetine Hydrochloride [orange Book]
87. (s)-duloxetine Hydrochloride, >=98% (hplc)
88. D01179
89. Duloxetine Hydrochloride [ep Monograph]
90. Duloxetine Hydrochloride [usp Monograph]
91. 434d349
92. Q-102508
93. Duloxetine Hydrochloride 1.0 Mg/ml In Methanol (as Free Base)
94. (+)-(s)-n-methyl-3-(1-naphthyloxy)-3-(2-thienyl)propanamine Hydrochloride
95. Duloxetine Hydrochloride, European Pharmacopoeia (ep) Reference Standard
96. (+)-(s)-n-methyl-3-(1-naphthyloxy)- 3-(2-thienyl)propanamine Hydrochloride
97. (3s)-n-methyl-3-(naphthalen-1-yloxy)-3-(2-thienyl)propan-1-amine Hydrochloride
98. (3s)-n-methyl-3-naphthalen-1-yloxy-3-thiophen-2-ylpropan-1-amin Hydrochloride.
99. (gammas)-2-thiophenepropanamine, N-methyl-gamma-(1-naphthalenyloxy)hydrochloride (1:1)
100. (gammas)-n-methyl-gamma-(1-naphthalenyloxy)-2-thiophenepropanamine Hydrochloride
101. (s)-(+)-n-methyl-3-(1-naphthalenyloxy)-3-(2-thienyl) Propanamine Hydrochloride
102. (s)-(+)-n-methyl-3-(1-naphthyloxy)-3-(2-thienyl)propylamine Hydrochloride
103. 2-thiophenepropanamine, N-methyl-.gamma.-(1-naphthalenyloxy)-, Hydrochloride, (s)-
104. Duloxetine For System Suitability, European Pharmacopoeia (ep) Reference Standard
105. Duloxetine Hydrochloride, Pharmaceutical Secondary Standard; Certified Reference Material
106. Duloxetine Hydrochloride, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 333.9 g/mol |
|---|---|
| Molecular Formula | C18H20ClNOS |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 6 |
| Exact Mass | 333.0954131 g/mol |
| Monoisotopic Mass | 333.0954131 g/mol |
| Topological Polar Surface Area | 49.5 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 312 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Cymbalta |
| PubMed Health | Duloxetine (By mouth) |
| Drug Classes | Antidepressant, Central Nervous System Agent, Neuropathic Pain Agent |
| Drug Label | Cymbalta (Duloxetine Delayed-Release Capsules) is a selective serotonin and norepinephrine reuptake inhibitor (SSNRI) for oral administration. Its chemical designation is (+)-( )- -methyl--(1-naphthyloxy)-2-thiophenepropylamine hydrochloride. The e... |
| Active Ingredient | Duloxetine hydrochloride |
| Dosage Form | Capsule, delayed rel pellets |
| Route | oral; Oral |
| Strength | eq 30mg base; eq 20mg base; eq 60mg base |
| Market Status | Prescription |
| Company | Lilly |
| 2 of 4 | |
|---|---|
| Drug Name | Duloxetine hydrochloride |
| Drug Label | Cymbalta (Duloxetine Delayed-Release Capsules) is a selective serotonin and norepinephrine reuptake inhibitor (SSNRI) for oral administration. Its chemical designation is (+)-( )- -methyl--(1-naphthyloxy)-2-thiophenepropylamine hydrochloride. The e... |
| Active Ingredient | Duloxetine hydrochloride |
| Dosage Form | Capsule, delayed rel pellets; Capsule, delayed release |
| Route | oral; Oral |
| Strength | eq 40mg base; 60mg; 30mg; eq 30mg base; eq 20mg base; eq 60mg base; 20mg |
| Market Status | Tentative Approval; Prescription |
| Company | Wockhardt; Actavis Elizabeth; Breckenridge Pharm; Apotex; Alembic Pharms; Teva Pharms Usa; Aurobindo Pharma; Torrent Pharms; Zydus Pharms Usa; Lupin; Dr Reddys Labs; Sandoz; Sun Pharma Global; Impax Labs |
| 3 of 4 | |
|---|---|
| Drug Name | Cymbalta |
| PubMed Health | Duloxetine (By mouth) |
| Drug Classes | Antidepressant, Central Nervous System Agent, Neuropathic Pain Agent |
| Drug Label | Cymbalta (Duloxetine Delayed-Release Capsules) is a selective serotonin and norepinephrine reuptake inhibitor (SSNRI) for oral administration. Its chemical designation is (+)-( )- -methyl--(1-naphthyloxy)-2-thiophenepropylamine hydrochloride. The e... |
| Active Ingredient | Duloxetine hydrochloride |
| Dosage Form | Capsule, delayed rel pellets |
| Route | oral; Oral |
| Strength | eq 30mg base; eq 20mg base; eq 60mg base |
| Market Status | Prescription |
| Company | Lilly |
| 4 of 4 | |
|---|---|
| Drug Name | Duloxetine hydrochloride |
| Drug Label | Cymbalta (Duloxetine Delayed-Release Capsules) is a selective serotonin and norepinephrine reuptake inhibitor (SSNRI) for oral administration. Its chemical designation is (+)-( )- -methyl--(1-naphthyloxy)-2-thiophenepropylamine hydrochloride. The e... |
| Active Ingredient | Duloxetine hydrochloride |
| Dosage Form | Capsule, delayed rel pellets; Capsule, delayed release |
| Route | oral; Oral |
| Strength | eq 40mg base; 60mg; 30mg; eq 30mg base; eq 20mg base; eq 60mg base; 20mg |
| Market Status | Tentative Approval; Prescription |
| Company | Wockhardt; Actavis Elizabeth; Breckenridge Pharm; Apotex; Alembic Pharms; Teva Pharms Usa; Aurobindo Pharma; Torrent Pharms; Zydus Pharms Usa; Lupin; Dr Reddys Labs; Sandoz; Sun Pharma Global; Impax Labs |
- Treatment of major depressive disorder;
- Treatment of diabetic peripheral neuropathic pain;
- Treatment of generalised anxiety disorder;
- Duloxetine Mylan is indicated in adults.
Duloxetine Lilly is indicated in adults for:
- Treatment of major depressive disorder
- Treatment of diabetic peripheral neuropathic pain
- Treatment of generalised anxiety disorder
Duloxetine Lilly is indicated in adults.
Yentreve is indicated for women for the treatment of moderate to severe stress urinary incontinence (SUI).
Treatment of major depressive disorder.
Treatment of diabetic peripheral neuropathic pain.
Treatment of generalised anxiety disorder.
Cymbalta is indicated in adults.
Treatment of diabetic peripheral neuropathic pain.
Ariclaim is indicated in adults.
Treatment of diabetic peripheral neuropathic pain in adults.
Treatment of chronic pain, Treatment of diabetic neuropathic pain, Treatment of generalised anxiety disorder, Treatment of major depressive disorder, Treatment of stress urinary incontinence
Antidepressive Agents
Mood-stimulating drugs used primarily in the treatment of affective disorders and related conditions. Several MONOAMINE OXIDASE INHIBITORS are useful as antidepressants apparently as a long-term consequence of their modulation of catecholamine levels. The tricyclic compounds useful as antidepressive agents (ANTIDEPRESSIVE AGENTS, TRICYCLIC) also appear to act through brain catecholamine systems. A third group (ANTIDEPRESSIVE AGENTS, SECOND-GENERATION) is a diverse group of drugs including some that act specifically on serotonergic systems. (See all compounds classified as Antidepressive Agents.)
Analgesics
Compounds capable of relieving pain without the loss of CONSCIOUSNESS. (See all compounds classified as Analgesics.)
Serotonin and Noradrenaline Reuptake Inhibitors
Drugs that selectively block or suppress the plasma membrane transport of SEROTONIN and NORADRENALINE into axon terminals and are used as ANTIDEPRESSIVE AGENTS. (See all compounds classified as Serotonin and Noradrenaline Reuptake Inhibitors.)
Dopamine Agents
Any drugs that are used for their effects on dopamine receptors, on the life cycle of dopamine, or on the survival of dopaminergic neurons. (See all compounds classified as Dopamine Agents.)
N06AX21
N06AX21
N06AX21
N06AX21
N06AX21
N06AX21
Registration Number : 302MF10013
Registrant's Address : Cesar Martinell i Brunet 12A, Poligono Rubi Sur, Rubi (Barcelona), Spain
Initial Date of Registration : 2020-01-27
Latest Date of Registration : 2020-01-27
 SCI Pharmtech offers high-quality, cost-effective APIs, advanced intermediates, & custom products with global expertise and precision.
SCI Pharmtech offers high-quality, cost-effective APIs, advanced intermediates, & custom products with global expertise and precision.
Registration Number : 229MF10017
Registrant's Address : No. 61, Ln. 309, Haihu N. Rd. , Luzhu Dist. , Taoyuan City 33856, Taiwan
Initial Date of Registration : 2017-01-25
Latest Date of Registration : 2017-01-25
Duloxetine hydrochloride "Teva"
Registration Number : 302MF10024
Registrant's Address : Neot-Hovav Eco-Industrial Park, Emek Sara P. O. Box 2049 Be'er Sheva 8412316, Israel
Initial Date of Registration : 2020-02-06
Latest Date of Registration : 2020-02-06

Registration Number : 302MF10002
Registrant's Address : 25, Barangongdan-ro, Hyangnam-eup, Hwaseong-si, Gyeonggi-do, Korea
Initial Date of Registration : 2020-01-07
Latest Date of Registration : 2020-01-07

Duloxetine hydrochloride (for manufacturing purposes only)
Registration Number : 305MF10047
Registrant's Address : 55 Yokohoonji, Kamiichi-cho, Nakaniikawa-gun, Toyama Prefecture
Initial Date of Registration : 2023-04-05
Latest Date of Registration : 2023-04-05

Registration Number : 301MF10085
Registrant's Address : 57, Gyeongje-ro, Siheung-si, Gyeonggi-do, Korea
Initial Date of Registration : 2019-10-25
Latest Date of Registration : 2019-11-27

Registration Number : 302MF10022
Registrant's Address : 7-2-A2, Hetero Corporate, Industrial Estates, Sanath Nagar, Hyderabad-500 018, Telang...
Initial Date of Registration : 2020-02-06
Latest Date of Registration : 2020-02-06

Registration Number : 302MF10025
Registrant's Address : Kalpataru Inspire, 3rd Floor, Off Western Express Highway, Santacruz (East), Mumbai 4...
Initial Date of Registration : 2020-02-06
Latest Date of Registration : 2020-11-18

Registration Number : 229MF10088
Registrant's Address : Plot No: C-24, Sanath Nagar Industrial Estate, Sanath Nagar, Hyderabad, Telangana, In...
Initial Date of Registration : 2017-04-26
Latest Date of Registration : 2022-11-16

Registration Number : 302MF10003
Registrant's Address : 1978-96 Ogushi, Ube City, Yamaguchi Prefecture
Initial Date of Registration : 2020-01-07
Latest Date of Registration : 2020-01-07

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?