
Synopsis
Synopsis
0
EU WC
0
VMF
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
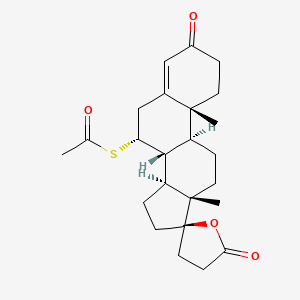

1. Aldactone
2. Aldactone A
3. Aquareduct
4. Ct, Spiro Von
5. Duraspiron
6. Espironolactona Alter
7. Espironolactona Mundogen
8. Flumach
9. Frumikal
10. Jenaspiron
11. Novo Spiroton
12. Novo-spiroton
13. Novospiroton
14. Practon
15. Sc 9420
16. Sc-9420
17. Sc9420
18. Spiractin
19. Spiro L.u.t.
20. Spiro Von Ct
21. Spirobeta
22. Spirogamma
23. Spirolactone
24. Spirolang
25. Spirono Isis
26. Spirono-isis
27. Spironone
28. Spirospare
29. Veroshpiron
30. Verospiron
31. Verospirone
32. Von Ct, Spiro
1. 52-01-7
2. Aldactone
3. Spirolactone
4. Verospirone
5. Verospiron
6. Euteberol
7. Spirolang
8. Spiroctan
9. Acelat
10. Aldactone A
11. Spironocompren
12. Melarcon
13. Spiresis
14. Spiridon
15. Uractone
16. Urusonin
17. Alderon
18. Spirone
19. Xenalon
20. Dira
21. Spiro-tablinen
22. Spironolactone A
23. Spironolattone
24. Aquareduct
25. Spironolactonum
26. Aldopur
27. Espironolactona
28. Abbolactone
29. Aldace
30. Almatol
31. Deverol
32. Altex
33. Berlactone
34. Sc 9420
35. Sc-9420
36. Spironolactone Ceva
37. Nsc-150399
38. Chembl1393
39. Chebi:9241
40. Spiro(17h-cyclopenta(a)phenauthrene-17,2'-(3'h)-furan)
41. Sc9420
42. Spironolattone [dcit]
43. 27o7w4t232
44. S-[(7r,8r,9s,10r,13s,14s,17r)-10,13-dimethyl-3,5'-dioxospiro[2,6,7,8,9,11,12,14,15,16-decahydro-1h-cyclopenta[a]phenanthrene-17,2'-oxolane]-7-yl] Ethanethioate
45. Aldactide
46. Spironolactonum [inn-latin]
47. Espironolactona [inn-spanish]
48. Dsstox_cid_14186
49. Dsstox_rid_79120
50. Dsstox_gsid_34186
51. Sc 15983
52. (1's,2r,2'r,9'r,10'r,11's,15's)-9'-(acetylsulfanyl)-2',15'-dimethylspiro[oxolane-2,14'-tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan]-6'-ene-5,5'-dione
53. 17-hydroxy-7-alpha-mercapto-3-oxo-17-alpha-pregn-4-ene-21-carboxylic Acid-gamma-lactone-7-acetate
54. S-[(7r,8r,9s,10r,13s,14s,17r)-10,13-dimethyl-3,5'-dioxo-1,2,3,4',5',6,7,8,9,10,11,12,13,14,15,16-hexadecahydro-3'h-spiro[cyclopenta[a]phenanthrene-17,2'-furan]-7-yl] Ethanethioate
55. Spiro[17h-cyclopenta[a]phenauthrene-17,2'-(3'h)-furan]
56. Diatense
57. Aldactone (tn)
58. Smr000471892
59. Hsdb 3184
60. Einecs 200-133-6
61. Mfcd00082250
62. Nsc 150399
63. Brn 0057767
64. Spirotone
65. Spiranolactone
66. Carospir
67. Unii-27o7w4t232
68. 2oax
69. 3vhu
70. Cas-52-01-7
71. Ncgc00015948-02
72. 3-(3-keto-7.alpha.-acetylthio-17.beta.-hydroxy-4-androsten-17.alpha.-yl)propionic Acid Lactone
73. 7-.alpha.-(acetylthio)-17-.alpha.-hydroxy-3-oxopregn-4-ene-21-carboxylic Acid, .gamma.-lactone
74. 7-alpha-acetylthio-3-oxo-17-alpha-pregn-4-ene-21,17-beta-carbolactone
75. S-((7r,8r,9s,10r,13s,14s,17r)-10,13-dimethyl-3,5'-dioxo-1,2,3,4',5',6,7,8,9,10,11,12,13,14,15,16-hexadecahydro-3'h-spiro[cyclopenta[a]phenanthrene-17,2'-furan]-7-yl) Ethanethioate
76. Spironolactone [usp:inn:ban:jan]
77. Pregn-4-ene-21-carboxylic Acid, 7-(acetylthio)-17-hydroxy-3-oxo-, Gamma-lactone, (7alpha,17alpha)-
78. Prestwick0_000128
79. Prestwick1_000128
80. Prestwick2_000128
81. Prestwick3_000128
82. Spironolactone [mi]
83. 17-hydroxy-7alpha-mercapto-3-oxo-17alpha-pregn-4-ene-21-carboxylic Acid, Gamma-lactone Acetate
84. 3'-(3-oxo-7-alpha-acetylthio-17-beta-hydroxyandrost-4-en-17-beta-yl)propionic Acid Lactone
85. 3-(3-keto-7-alpha-acetylthio-17-beta-hydroxy-4-androsten-17-alpha-yl)propionic Acid Lactone
86. Spironolactone [inn]
87. Spironolactone [jan]
88. Bidd:pxr0071
89. Schembl20939
90. Bspbio_000176
91. Spironolactone [hsdb]
92. Spironolactone [iarc]
93. Spironolactone [inci]
94. 17alpha-pregn-4-ene-21-carboxylic Acid, 17-hydroxy-7alpha-mercapto-3-oxo-, Gamma-lactone, Acetate
95. 4-18-00-01601 (beilstein Handbook Reference)
96. Mls001074672
97. Mls001333253
98. Mls001333254
99. Mls002153245
100. Mls002207058
101. Mls002548846
102. Spironolactone [vandf]
103. Spbio_002115
104. Spironolactone [mart.]
105. Bpbio1_000194
106. Gtpl2875
107. Spironolactone [usp-rs]
108. Spironolactone [who-dd]
109. Spironolactone [who-ip]
110. Dtxsid6034186
111. Spironolactone (jp17/usp/inn)
112. Hms1568i18
113. Hms2090n21
114. Hms2095i18
115. Hms2236e06
116. Hms3259g11
117. Hms3712i18
118. Act02596
119. Amy40521
120. Hy-b0561
121. Spironolactone, 97.0-103.0%
122. Zinc3861599
123. Spironolactone [orange Book]
124. Tox21_113047
125. Tox21_302154
126. 7alpha-(acetylsulfanyl)-3-oxo-17alpha-pregn-4-ene-21,17-carbolactone
127. Bdbm50228080
128. Nsc150399
129. S4054
130. Spironolactone [ep Monograph]
131. Spironolactone [usp Monograph]
132. Akos015896401
133. Spironolactonum [who-ip Latin]
134. Ac-4214
135. Ccg-220128
136. Db00421
137. Ks-5234
138. Nc00482
139. 7-alpha-(acetylthio)-17-alpha-hydroxy-3-oxopregn-4-ene-21-carboxylic Acid, Gamma-lactone
140. Cardalis Component Spironolactone
141. Spironolactone Component Cardalis
142. Ncgc00164397-01
143. Ncgc00164397-02
144. Ncgc00164397-03
145. Ncgc00164397-05
146. Ncgc00255229-01
147. 17-alpha-pregn-4-ene-21-carboxylic Acid, 17-hydroxy-7-alpha-mercapto-3-oxo-, Gamma-lactone Acetate
148. Bs166385
149. Cpd000471892
150. Spironolactone 1.0 Mg/ml In Acetonitrile
151. Aldactazide Component Spironolactone
152. Spironolactone [ema Epar Veterinary]
153. Ab00513806
154. S0260
155. Spironolactone Component Of Aldactazide
156. C07310
157. C75438
158. D00443
159. Ab01275520-01
160. Ab01275520_02
161. 082s250
162. Q422188
163. Sr-01000765419
164. Sr-05000000452
165. Q-201737
166. Sr-01000765419-2
167. Sr-05000000452-2
168. Brd-k90027355-001-03-4
169. Brd-k90027355-001-19-0
170. Z1551900341
171. Spironolactone, European Pharmacopoeia (ep) Reference Standard
172. Wln: L E5 B666 Fx Ov Mutj A1 E1 Ksv1 F-& Ct5voxtj
173. 17.alpha.-pregn-4-ene-21-carboxylic Acid, .gamma.-lactone Acetate
174. Spironolactone, United States Pharmacopeia (usp) Reference Standard
175. 17.alpha.-pregn-4-ene-21-carboxylic Acid, .gamma.-lactone, Acetate
176. Pregn-4-ene-21-carboxylic Acid, .gamma.-lactone, (7.alpha.,17.alpha.)-
177. (7?,17?)-7-(acetylthio)-17-hydroxy-3-oxopregn-4-ene-21-carboxylic Acid ?-lactone
178. Spironolactone For System Suitability, European Pharmacopoeia (ep) Reference Standard
179. 17-hydroxy-7.alpha.-mercapto-3-oxo-17.alpha.-pregn-4-ene-21-carboxylic Acid, .gamma.-lactone Acetate
180. 17-hydroxy-7alpha-mercapto-3-oxo-17alpha-pregn-4-ene-21- Carboxylic Acid; Gamma-lactone Acetate
181. 2'',15''-dimethyl-5,5''-dioxo-(9''r)-spiro[tetrahydrofuran-2,14''-tetracyclo[8.7.0.02,7.011,15]heptadec-6''-ene]-9-yl Ethanethioate
182. 2'',15''-dimethyl-5,5''-dioxospiro[tetrahydrofuran-2,14''-tetracyclo[8.7.0.02,7.011,15]heptadec-6''-ene]-9-yl Ethanethioate
183. 2'',15''-dimethyl-5,5''-dioxospiro[tetrahydrofuran-2,14''-tetracyclo[8.7.0.02,7.011,15]heptadec-6''-ene]-9-yl Ethanethioate(spiranolactone)
184. Pregn-4-ene-21-carboxylic Acid, 7-(acetylthio)-17-hydroxy-3-oxo-,.gamma.-lactone, (7.alpha.,17.alpha.)-
185. S-(2''r,7r,8r,9s,10r,13s,14s)-10,13-dimethyl-3,5''-dioxo-1,2,3,4'',5'',6,7,8,9,10,11,12,13,14,15,16-hexadecahydro-3''h-spiro[cyclopenta[a]phenanthrene-17,2''-furan]-7-yl Ethanethioate
| Molecular Weight | 416.6 g/mol |
|---|---|
| Molecular Formula | C24H32O4S |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Exact Mass | 416.20213067 g/mol |
| Monoisotopic Mass | 416.20213067 g/mol |
| Topological Polar Surface Area | 85.7 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 818 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Aldactazide |
| PubMed Health | Spironolactone/Hydrochlorothiazide (By mouth) |
| Drug Classes | Diuretic, Potassium Sparing/Thiazide Combination |
| Active Ingredient | Hydrochlorothiazide; spironolactone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 50mg |
| Market Status | Prescription |
| Company | Gd Searle |
| 2 of 6 | |
|---|---|
| Drug Name | Aldactone |
| PubMed Health | Spironolactone (By mouth) |
| Drug Classes | Antiandrogen, Cardiovascular Agent |
| Drug Label | ALDACTONE oral tablets contain 25 mg, 50 mg, or 100 mg of the aldosterone antagonist spironolactone, 17-hydroxy-7-mercapto-3-oxo-17-pregn-4-ene-21-carboxylic acid -lactone acetate, which has the following structural formula:Spironolactone is pr... |
| Active Ingredient | Spironolactone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg; 25mg; 50mg |
| Market Status | Prescription |
| Company | Gd Searle |
| 3 of 6 | |
|---|---|
| Drug Name | Spironolactone |
| PubMed Health | Spironolactone (By mouth) |
| Drug Classes | Antiandrogen, Cardiovascular Agent |
| Drug Label | Spironolactone Tablets, USP contain 25 mg, 50 mg, or 100 mg of the aldosterone antagonist spironolactone, 17-hydroxy-7acidacetate, which has the following structural formula:Spironolactone is practically insoluble in water, soluble in alcohol, and fr... |
| Active Ingredient | Spironolactone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Amneal Pharms; Sandoz; Orion Corp Orion; Actavis Elizabeth; Jubilant Generics; Mutual Pharm; Vintage; Mylan |
| 4 of 6 | |
|---|---|
| Drug Name | Aldactazide |
| PubMed Health | Spironolactone/Hydrochlorothiazide (By mouth) |
| Drug Classes | Diuretic, Potassium Sparing/Thiazide Combination |
| Active Ingredient | Hydrochlorothiazide; spironolactone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 50mg |
| Market Status | Prescription |
| Company | Gd Searle |
| 5 of 6 | |
|---|---|
| Drug Name | Aldactone |
| PubMed Health | Spironolactone (By mouth) |
| Drug Classes | Antiandrogen, Cardiovascular Agent |
| Drug Label | ALDACTONE oral tablets contain 25 mg, 50 mg, or 100 mg of the aldosterone antagonist spironolactone, 17-hydroxy-7-mercapto-3-oxo-17-pregn-4-ene-21-carboxylic acid -lactone acetate, which has the following structural formula:Spironolactone is pr... |
| Active Ingredient | Spironolactone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg; 25mg; 50mg |
| Market Status | Prescription |
| Company | Gd Searle |
| 6 of 6 | |
|---|---|
| Drug Name | Spironolactone |
| PubMed Health | Spironolactone (By mouth) |
| Drug Classes | Antiandrogen, Cardiovascular Agent |
| Drug Label | Spironolactone Tablets, USP contain 25 mg, 50 mg, or 100 mg of the aldosterone antagonist spironolactone, 17-hydroxy-7acidacetate, which has the following structural formula:Spironolactone is practically insoluble in water, soluble in alcohol, and fr... |
| Active Ingredient | Spironolactone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Amneal Pharms; Sandoz; Orion Corp Orion; Actavis Elizabeth; Jubilant Generics; Mutual Pharm; Vintage; Mylan |
Aldosterone Antagonists; Diuretics
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
/EXPL THER:/ Aldosterone is important in the pathophysiology of heart failure. In a doubleblind study, we enrolled 1663 patients who had severe heart failure and a left ventricular ejection fraction of no more than 35 percent and who were being treated with an angiotensin-converting-enzyme inhibitor, a loop diuretic, and in most cases digoxin. A total of 822 patients were randomly assigned to receive 25 mg of spironolactone daily, and 841 to receive placebo. The primary end point was death from all causes. The trial was discontinued early, after a mean follow-up period of 24 months, because an interim analysis determined that spironolactone was efficacious. There were 386 deaths in the placebo group (46 percent) and 284 in the spironolactone group (35 percent; relative risk of death, 0.70; 95 percent confidence interval, 0.60 to 0.82; P<0.001). This 30 percent reduction in the risk of death among patients in the spironolactone group was attributed to a lower risk of both death from progressive heart failure and sudden death from cardiac causes. The frequency of hospitalization for worsening heart failure was 35 percent lower in the spironolactone group than in the placebo group (relative risk of hospitalization, 0.65; 95 percent confidence interval, 0.54 to 0.77; P<0.001). In addition, patients who received spironolactone had a significant improvement in the symptoms of heart failure, as assessed on the basis of the New York Heart Association functional class (P<0.001). Gynecomastia or breast pain was reported in 10 percent of men who were treated with spironolactone, as compared with 1 percent of men in the placebo group (P<0.001). The incidence of serious hyperkalemia was minimal in both groups of patients.
PMID:10471456 Pitt B et al; N Engl J Med 341 (10): 709-17 (1999)
MEDICATION (VET): Spironolactone can be used with furosemide to control ascites.
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 380
MEDICATION (VET): Spironolactone is used most frequently and is a competitive antagonist of aldosterone. Aldosterone is elevated in animals with congestive heart failure in which the renin-angiotensin system is activated in response to hyponatremia, hyperkalemia, and reductions in blood pressure or cardiac output. Aldosterone is responsible for increasing sodium and chloride reabsorption and potassium and calcium excretion from renal tubules. Spironolactone competes with aldosterone at its receptor site, causing a mild diuresis and potassium retention.
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2046
For more Therapeutic Uses (Complete) data for SPIRONOLACTONE (12 total), please visit the HSDB record page.
Spironolactone is an aldosterone antagonist that acts on the mineralocorticoid receptor. It is a potassium-sparing diuretic, and hyperkalemia is the most common and potentially serious complication of therapy. Impaired kidney function appears to increase this risk, as does supplementation with potassium chloride. Excessive diuresis can also lead to dehydration and hyponatraemia. A number of endocrine effects have also been reported, the most common of which is gynaecomastia, with a dose-related incidence of 7-52%. This side-effect is reversible and disappears upon discontinuation of therapy. Other endocrine effects include loss of sexual potency in men and menstrual irregularity, amenorrhea, breast engorgement and chloasma in women. These effects are probably due to interaction of spironolactone with the androgen receptor.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V79 327 (2001)
There are a few isolated case reports of idiosyncratic drug reactions, including one case of hepatitis and several cases of agranulocytosis. Approximately 10 cases have been reported of allergic contact dermatitis after topical application of spironolactone for various dermal indications involving its antiandrogen activity.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V79 327 (2001)
Maternal Medication usually Compatible with Breast-Feeding: Spironolactone: Reported Sign or Symptom in Infant or Effect on Lactation: None. /From Table 6/
Report of the American Academy of Pediatrics Committee on Drugs in Pediatrics 93 (1): 142 (1994)
POTENTIAL ADVERSE EFFECTS ON FETUS: May cross placenta. No controlled studies performed, but no known teratogenic effects. POTENTIAL SIDE EFFECTS ON BREAST-FED INFANT: Active metabolite (canrenone) excreted in breast milk. FDA Category: C (C = Studies in laboratory animals have revealed adverse effects on the fetus (teratogenic, embryocidal, etc.) but there are no controlled studies in pregnant women. The benefits from use of the drug in pregnant women may be acceptable despite its potential risks, or there are no laboratory animal studies or adequate studies in pregnant women.) /From table II/
PMID:2195076 Stockton DL, Paller AS; J Am Acad Dermatol 23 (1):87-103 (1990)
For more Drug Warnings (Complete) data for SPIRONOLACTONE (17 total), please visit the HSDB record page.
Spironolactone is indicated for the treatment of New York Heart Association Class III-IV heart failure, management of edema in cirrhotic adults not responsive to fluid and sodium restrictions, primary hyperaldosteronism short-term preoperatively, primary hyperaldosteronism long-term in patients with aldosterone producing adrenal adenomas that are not candidates for surgery or patients with bilarteral micro/macronodular adrenal hyperplasia, as an add-on therapy in hypertension, and in nephrotic syndrome when treatment of the disease as well as fluid and sodium restriction with other diuretics is inadequate. Spironolactone has antiandrogenic activity which leads to many of its off label uses. Spironolactone is used off label in the treatment of hirsutism, female pattern hair loss, and adult acne vulgaris. Spironolactone is also frequently used for its antiandrogenic effects in transgender female patients due to its low cost and reducing male-pattern hair growth.
FDA Label
For use in combination with standard therapy (including diuretic support, where necessary) for the treatment of congestive heart failure caused by valvular regurgitation in dogs.
Originally spironolactone was only studied for its potassium sparing diuretic effect. Spironolactone competitively inhibits mineralocorticoid receptors in the distal convoluted tubule to promote sodium and water excretion and potassium retention.. Inhibition of this receptor leads to increased renin and aldosterone levels. Spironolactone is structurally similar to progesterone and as a result is associated with progestogenic and antiandrogenic effects.
Mineralocorticoid Receptor Antagonists
Drugs that bind to and block the activation of MINERALOCORTICOID RECEPTORS by MINERALOCORTICOIDS such as ALDOSTERONE. (See all compounds classified as Mineralocorticoid Receptor Antagonists.)
Diuretics
Agents that promote the excretion of urine through their effects on kidney function. (See all compounds classified as Diuretics.)
QC03DA01
C03DA01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C03 - Diuretics
C03D - Aldosterone antagonists and other potassium-sparing agents
C03DA - Aldosterone antagonists
C03DA01 - Spironolactone
Absorption
Spironolactone reaches a maximum concentration in 2.6 hours and an active metabolite (canrenone) reaches a maximum concentration in 4.3 hours. When taken with food, the bioavailability of spironolactone increases to 95.4%. Giving spironolactone with food increases the maximum concentration from 209ng/mL to 301ng/mL. The time to maximum concentration also increases from 2.28 hours to 3.05 hours. The area under the curve varies from 2103ng/mL\*hr to 4544ng/mL*hr.
Route of Elimination
Metabolites of spironolactone are excreted 42-56% in urine, and 14.2-14.6% in the feces. No unmetabolized spironolactone is present in the urine.
Volume of Distribution
Volume of distribution data is not readily available.
Clearance
Clearance data is not readily available.
At the time spironolactone was introduced for clinical use, its bioavailability was inadequate, and this was improved by preparing the drug in finely powdered or micronized form. The absolute bioavailability was indirectly estimated at approximately 73%, which was enhanced in the presence of food. Nearly all absorbed spironolactone (> 90 %) is bound to plasma proteins and, with repeated dosing, a steady state is achieved within 8 days. After oral intake of a 100-mg dose, the plasma half-time of spironolactone was 1-2 h, the time to maximum plasma concentration was 2-3.2 hr, the maximum blood concentration was 92-148 ng/mL, the area under the concentration--time (0-24 hr) curve was 1430-1541 ng/mL per hr and the elimination half-time was 18-20 hr.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V79 325 (2001)
The disposition of (14)C spironolactone was studied in male rats, female dogs and female monkeys after intravenous or oral administration of 5 mg/kg bw. Gastrointestinal absorption was estimated to be 82% in rats, 62% in dogs and 103% in monkeys. Spironolactone was extensively metabolized in all three species, and the metabolites were excreted primarily in the urine and feces. The amount of radiolabel excreted in urine or feces of all three species was similar after intravenous and after oral dosing. In monkeys, as in humans, the amounts excreted in urine and feces were about equal, while fecal excretion predominated in rats and dogs as a result of biliary excretion. After the oral dose, the percentage of urinary excretion was 4.7% in rats, 18% in dogs and 46% in monkeys. The high excretion of radiolabel in the feces of rats (90%) after intravenous administration shows the importance of biliary excretion for that species. Species differences were also noted in the biotransformation of spironolactone. ...
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V79 326 (2001)
Absorption of spironolactone from the GI tract depends on the formulation in which it is administered. Currently available formulations of spironolactone are well absorbed from the GI tract and bioavailability of the drug exceeds 90% when compared to an optimally absorbed spironolactone solution in polyethylene glycol 400. Following a single oral dose of spironolactone, peak serum concentrations of the drug occur within 1-2 hours, and peak serum concentrations of its principal metabolites are attained within 2-4 hours. When spironolactone is administered concomitantly with food, peak serum concentrations and areas under the serum concentration-time curves (AUCs) of the drug and, to a lesser degree, its principal metabolites are increased substantially compared with the fasting state...
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 1983
Spironolactone and canrenone, a major metabolite of the drug, are both more than 90% bound to plasma proteins. Spironolactone or its metabolites may cross the placenta. Canrenone, a major metabolite of spironolactone, is distributed into milk.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 1983
Spironolactone is deacetylated to 7-thiospironolactone. 7-thiospironolactone is S-methylated to 7-thiomethylspironolactone or undergoes an elimination reaction to canrenone. 7-thiomethylspironolactone is reduced to 3-hydroxythiomethylspironolactone or 3-hydroxythiomethylspironolactone. Canrenone was originally thought to be the primary circulating metabolite, however more recent studies have demonstrated that the primary metabolite is actually 7-thiomethylspironolactone.
Spironolactone is rapidly and extensively metabolized to compounds that are excreted in the urine and faeces. It undergoes enterohepatic recirculation, but no unchanged drug appears in urine or feces. The metabolites of spironolactone can be divided into two main groups: those in which the sulfur moiety is retained and those in which the sulfur is removed by dethioacetylation. For many years, it was thought that the dethioacetylated metabolite, canrenone, was the major metabolite; however, with more specific analytical methods such as HPLC, 7alpha-thiomethylspirolactone was recognized as the major metabolite of spironolactone. This metabolite is formed by hydrolysis of the thioacetate group to form 7alpha-thiospirolactone (as an intermediate), followed by S-methylation to 7alpha-thiomethylspirolactone. This can then be hydroxylated to form 6-hydroxy-7alpha-thiomethylspirolactone and oxidized to form 7alpha-methylsulfinyl- and 7alpha-methylsulfonylspirolactone or via sulfoxidation to form 6alpha-hydroxy-7alpha-methylsulfinyl- and 6beta-hydroxy-7alpha-methylsulfonylspirolactone. For formation of the group of metabolites in which sulfur is removed, 7alpha-thiomethylspirolactone is also dethioacetylated to canrenone, which is further metabolized by three pathways: hydrolysis of the gamma-lactone ring to form canrenoate, which is excreted in the urine as a glucuronic ester, and, next, hydroxylation to form 15alpha-hydroxy-canrenone or reduction to produce several di-, tetra- and hexa-hydro derivatives. Canrenone and canrenoate are in equilibrium with one another. Not only spironolactone but several of its metabolites have biological activity; in decreasing order of potency, these are 7alpha-thiospirolactone, 7alpha-thiomethylspirolactone and canrenone.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V79 325 (2001)
Species differences were ... noted in the biotransformation of spironolactone. Canrenone was a principal extractable metabolite in rat and dog plasma, whereas in monkeys and humans, both canrenone and a very polar, unidentified metabolite were the major constituents. In the urine of all four species, canrenone was a principal constituent. Notable species differences in the metabolites of spironolactone in the feces were found, the pattern of metabolites in dog feces being markedly different from that in rats, monkeys or humans. Overall, it was concluded that the disposition and metabolism of spironolactone in monkeys, rather than that in rats or dogs, is closest to that in humans.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V79 326 (2001)
SIX METABOLITES OF SPIRONOLACTONE ... /HAVE/ BEEN DETECTED IN URINE OF TREATED SUBJECTS. ... /ONE IS/ DETHIOACETYLATED COMPD CANRENONE, 3-(3-OXO-17BETA-HYDROXY-4,6-ANDRO-STADIEN-17ALPHA-YL)PROPIONIC ACID GAMMA-LACTONE...
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 287
A FLUOROMETRIC METHOD WAS USED TO DETERMINE CONCN OF CANRENONE IN MILK FOLLOWING INGESTION OF SPIRONOLACTONE (ALDACTONE) 25 MG TWICE DAILY IN 28-YR-OLD FEMALE.
PMID:894512 PHELPS DL, KARIM A; J PHARM SCI 66: 1203 (1977)
1.4 hours. Canrenone has a half life of 16.5 hours, 7--thiomethylspirolactone has a half life of 13.8 hours, and 6--hydroxy-7--thiomethylspirolactone has a half life of 15 hours.
... Nearly all absorbed spironolactone (> 90 %) is bound to plasma proteins and, with repeated dosing, a steady state is achieved within 8 days. After oral intake of a 100-mg dose, the plasma half-time of spironolactone was 1-2 h, the time to maximum plasma concentration was 2-3.2 hr, the maximum blood concentration was 92-148 ng/mL, the area under the concentration--time (0-24 hr) curve was 1430-1541 ng/mL per hr and the elimination half-time was 18-20 hr.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V79 325 (2001)
Following a single oral dose in healthy adults, the half-life of spironolactone averages 1.3-2 hours, and the half-life of 7alpha-thiomethylspironolactone averages 2.8 hours. The half-life of canrenone reportedly ranges from 13-24 hours. In multiple-dose studies, the steady-state plasma elimination half-life of canrenone averaged 19.2 hours when 200 mg of spironolactone was administered daily as a single dose and averaged 12.5 hours when 200 mg of the drug was administered daily in 4 equally divided doses.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 1983
Spironolactone competitively inhibits aldosterone dependant sodium potassium exchange channels in the distal convoluted tubule. This action leads to increased sodium and water excretion, but more potassium retention. The increased excretion of water leads to diuretic and also antihypertensive effects.
Spironolactone can exert antiandrogenic effects by several mechanisms: it can destroy testicular CYP and decrease 17alpha-hydroxylase activity, resulting in decreased testosterone synthesis; and it can inhibit 5alpha-dihydrotestosterone binding to cytosolic androgen receptor in the prostate.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V79 328 (2001)
Spironolactone exhibits antiandrogenic effects in males and females. The mechanism of antiandrogenic activity of spironolactone is complex and appears to involve several effects of the drug. Spironolactone decreases testosterone biosynthesis by inhibiting steroid 17alpha-monooxygenase (17alpha-hydroxylase) activity, possibly secondary to destruction of microsomal cytochrome P-450 in tissues with high steroid 17alpha-monooxygenase activity (e.g., testes, adrenals). The drug also appears to competitively inhibit binding of dihydrotestosterone to its cytoplasmic receptor protein, thus decreasing androgenic actions at target tissues. Spironolactone-induced increases in serum estradiol concentration also may contribute to its antiandrogenic activity, although such increases may not occur consistently; such increases appear to result from increased conversion of testosterone to estradiol. Spironolactone may have variable effects on serum 17-hydroxyprogesterone concentrations, possibly decreasing its production by inhibiting steroid 17alpha-monooxygenase activity or decreasing its conversion (with resultant accumulation) to androstenedione by inhibiting cytochrome P450-dependent 17alpha-hydroxyprogesterone aldolase (17,20-desmolase) activity. Serum progesterone concentrations may increase with the drug secondary to decreased hydroxylation (via steroid 17alpha-monooxygenase) to 17-hydroxyprogesterone. In children, compensatory increases in lutropin (luteinizing hormone, LH) and follicle-stimulating hormone (FSH) secretion can occur, probably secondary to the drug's antiandrogenic effects (i.e., a feedback response to decreasing serum testosterone concentrations and/or peripheral androgenic activity).
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 1983
MEDICATION (VET): Spironolactone is used most frequently and is a competitive antagonist of aldosterone. Aldosterone is elevated in animals with congestive heart failure in which the renin-angiotensin system is activated in response to hyponatremia, hyperkalemia, and reductions in blood pressure or cardiac output. Aldosterone is responsible for increasing sodium and chloride reabsorption and potassium and calcium excretion from renal tubules. Spironolactone competes with aldosterone at its receptor site, causing a mild diuresis and potassium retention.
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2046
High concentrations of spironolactone have been reported to interfere with steroid biosynthesis by inhibiting 11 beta- and 18-, 21-, and 17 alpha-hydroxylase.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 780

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
91
PharmaCompass offers a list of Spironolactone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Spironolactone manufacturer or Spironolactone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Spironolactone manufacturer or Spironolactone supplier.
PharmaCompass also assists you with knowing the Spironolactone API Price utilized in the formulation of products. Spironolactone API Price is not always fixed or binding as the Spironolactone Price is obtained through a variety of data sources. The Spironolactone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A duraspiron manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of duraspiron, including repackagers and relabelers. The FDA regulates duraspiron manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. duraspiron API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of duraspiron manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A duraspiron supplier is an individual or a company that provides duraspiron active pharmaceutical ingredient (API) or duraspiron finished formulations upon request. The duraspiron suppliers may include duraspiron API manufacturers, exporters, distributors and traders.
click here to find a list of duraspiron suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A duraspiron DMF (Drug Master File) is a document detailing the whole manufacturing process of duraspiron active pharmaceutical ingredient (API) in detail. Different forms of duraspiron DMFs exist exist since differing nations have different regulations, such as duraspiron USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A duraspiron DMF submitted to regulatory agencies in the US is known as a USDMF. duraspiron USDMF includes data on duraspiron's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The duraspiron USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of duraspiron suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The duraspiron Drug Master File in Japan (duraspiron JDMF) empowers duraspiron API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the duraspiron JDMF during the approval evaluation for pharmaceutical products. At the time of duraspiron JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of duraspiron suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a duraspiron Drug Master File in Korea (duraspiron KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of duraspiron. The MFDS reviews the duraspiron KDMF as part of the drug registration process and uses the information provided in the duraspiron KDMF to evaluate the safety and efficacy of the drug.
After submitting a duraspiron KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their duraspiron API can apply through the Korea Drug Master File (KDMF).
click here to find a list of duraspiron suppliers with KDMF on PharmaCompass.
A duraspiron CEP of the European Pharmacopoeia monograph is often referred to as a duraspiron Certificate of Suitability (COS). The purpose of a duraspiron CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of duraspiron EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of duraspiron to their clients by showing that a duraspiron CEP has been issued for it. The manufacturer submits a duraspiron CEP (COS) as part of the market authorization procedure, and it takes on the role of a duraspiron CEP holder for the record. Additionally, the data presented in the duraspiron CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the duraspiron DMF.
A duraspiron CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. duraspiron CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of duraspiron suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing duraspiron as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for duraspiron API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture duraspiron as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain duraspiron and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a duraspiron NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of duraspiron suppliers with NDC on PharmaCompass.
duraspiron Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of duraspiron GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right duraspiron GMP manufacturer or duraspiron GMP API supplier for your needs.
A duraspiron CoA (Certificate of Analysis) is a formal document that attests to duraspiron's compliance with duraspiron specifications and serves as a tool for batch-level quality control.
duraspiron CoA mostly includes findings from lab analyses of a specific batch. For each duraspiron CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
duraspiron may be tested according to a variety of international standards, such as European Pharmacopoeia (duraspiron EP), duraspiron JP (Japanese Pharmacopeia) and the US Pharmacopoeia (duraspiron USP).
