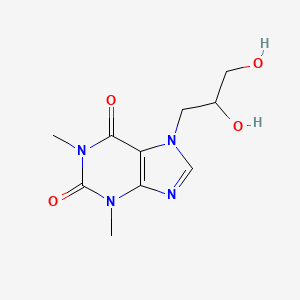

1. Dihydroxypropyltheophylline
2. Dilin
3. Diphylline
4. Diprophylline
5. Dylix
6. Lufyllin
7. Neothylline
1. Diprophylline
2. 479-18-5
3. 7-(2,3-dihydroxypropyl)theophylline
4. Lufyllin
5. Neothylline
6. Diprophyllin
7. Glyphylline
8. Aristophyllin
9. Diprofilline
10. Dipropylline
11. Neutrafillina
12. Neutraphyllin
13. Neutraphylline
14. Neutroxantina
15. Propyphyllin
16. Protheophylline
17. Synthophylline
18. Corphyllin
19. Diprofillin
20. Glyphyllin
21. Neophyllin
22. Neophyl
23. Dilor
24. Hidroxiteofillina
25. Neostenovasan
26. Silbephylline
27. Asthmolysin
28. Astrophyllin
29. Coronarin
30. Glyfyllin
31. Hiphyllin
32. Hyphylline
33. Liactemin
34. Neotilina
35. Neutrafil
36. Purifilin
37. Silbephyllin
38. Solufilin
39. Solufyllin
40. Soluphyllin
41. Thefylan
42. Circain
43. Coronal
44. Droxine
45. Dyflex
46. Neufil
47. Tefilan
48. Teofen
49. Theal
50. Neo-vasophylline
51. Cor-theophylline
52. Afi-phyllin
53. Dihydroxypropyl Theophylline
54. Diprophyllinum
55. Diprofilina
56. Dihydroxypropyl Theopylin
57. (1,2-dihydroxy-3-propyl)thiophyllin
58. Iphyllin
59. 7-(2,3-dihydroxypropyl)-1,3-dimethylxanthine
60. 7-(2,3-dihydroxypropyl)-1,3-dimethylpurine-2,6-dione
61. 7-(2,3-dioxypropyl)theophylline
62. 7-(beta,gamma-dihydroxypropyl)theophylline
63. Dyphilline
64. 1,3-dimethyl-7-(2,3-dihydroxypropyl)xanthine
65. Mfcd00005759
66. 7-(2,3-dihydroxypropyl)-1,3-dimethyl-3,7-dihydro-1h-purine-2,6-dione
67. Dyphylline (usp)
68. Dyphylline [usp]
69. 7-[2,3-dihydroxypropyl]-theophylline
70. 1h-purine-2,6-dione, 7-(2,3-dihydroxypropyl)-3,7-dihydro-1,3-dimethyl-
71. Diprophylline [inn]
72. 7-(2,3-dihydroxypropyl)-3,7-dihydro-1,3-dimethyl-1h-purine-2,6-dione
73. (+-)-7-(2,3-dihydroxypropyl)theophylline
74. Nsc-14305
75. Mls000069403
76. Chebi:4728
77. Astmamasit
78. Glyphyllinum
79. Isophyllen
80. Circair
81. Tesfen
82. Theal Ampules
83. Neophyllin M
84. 263t0e9rr9
85. 7-(2,3-dihydroxypropyl)-1,3-dimethyl-1h-purine-2,6(3h,7h)-dione
86. Diphyllin (van)
87. Diprofillina
88. Smr000059068
89. Diprofillina [dcit]
90. (+/-)-7-(2,3-dihydroxypropyl)theophylline
91. Dilor-400
92. Dsstox_cid_2975
93. 7-(2,3-dimethylxanthine
94. Dsstox_rid_76813
95. Dsstox_gsid_22975
96. 1,3-dihydroxypropyl)xanthine
97. Diprofilina [inn-spanish]
98. Diprophyllinum [inn-latin]
99. Theophylline,3-dihydroxypropyl)-
100. Dihydroxypropyl Theopylin (german)
101. 7-(2,3-dihydroxypropyl)-theophylline
102. Lufyllin (tn)
103. (+-)-dyphylline
104. (+-)-diprophylline
105. Hsdb 3322
106. 7-(.beta.,.gamma.-dihydroxypropyl)theophylline
107. Wln: T56 Bn Dn Fnvnvj B1yq1q F1 H1
108. Sr-01000002972
109. Einecs 207-526-1
110. Nsc 14305
111. Diprophylline (jan/inn)
112. Brn 0284563
113. Theophylline, 7-(2,3-dihydroxypropyl)-
114. Unii-263t0e9rr9
115. 1h-purine-2, 7-(2,3-dihydroxypropyl)-3,7-dihydro-1,3-dimethyl-
116. Ncgc00016455-01
117. Diprophylline,(s)
118. 1h-purine-2,6-dione, 7-(2,3-dihydroxypropyl)-3,7-dihydro-1,3-dimethyl
119. Cas-479-18-5
120. Prestwick_465
121. Dyphylline (dilor)
122. (+/-)-dyphylline
123. (+/-)-diprophylline
124. Spectrum_000809
125. Dyphylline [mi]
126. Opera_id_1754
127. Prestwick0_000033
128. Prestwick1_000033
129. Prestwick2_000033
130. Prestwick3_000033
131. Spectrum2_000090
132. Spectrum3_000411
133. Spectrum4_000530
134. Spectrum5_000953
135. Dyphylline [hsdb]
136. Dyphylline [inci]
137. Dyphylline [vandf]
138. 7-(2,3-dihydroxypropyl)-1,3-dimethyl-2,3,6,7-tetrahydro-1h-purine-2,6-dione
139. Diprophylline [jan]
140. Schembl8192
141. Chembl1752
142. Dyphylline [usp-rs]
143. Oprea1_363458
144. Bspbio_000125
145. Bspbio_001962
146. Kbiogr_001139
147. Kbioss_001289
148. 5-26-14-00070 (beilstein Handbook Reference)
149. Mls001076466
150. Diprophylline [mart.]
151. Divk1c_000022
152. Spectrum1500269
153. Spbio_000020
154. Spbio_002046
155. Diprophylline [who-dd]
156. Bpbio1_000139
157. Gtpl7070
158. Dtxsid6022975
159. Dyphylline [orange Book]
160. Bdbm82016
161. Hms500b04
162. Kbio1_000022
163. Kbio2_001289
164. Kbio2_003857
165. Kbio2_006425
166. Kbio3_001182
167. Kscfjbixmnovsh-uhfffaoysa-
168. Ninds_000022
169. Dyphylline [usp Monograph]
170. Hms1568g07
171. Hms1920k08
172. Hms2091b13
173. Hms2095g07
174. Hms2232k09
175. Hms3372h13
176. Hms3654d18
177. Hms3712g07
178. Hms3884e19
179. Pharmakon1600-01500269
180. Bcp02003
181. Hy-b0128
182. Nsc_3182
183. Nsc14305
184. Nsc40844
185. Diprophylline [ep Monograph]
186. Tox21_110444
187. Ac7833
188. Bbl009638
189. Ccg-38906
190. Nsc-40844
191. Nsc756753
192. S1504
193. Stk796769
194. Akos005203076
195. Tox21_110444_1
196. Bcp9000631
197. Cs-1901
198. Db00651
199. Nsc-756753
200. 1h-purine-2,6-dione, 7-(2,3-dihydroxypropyl)-3,7-dihydro-1,3-dimethyl-, (+-)-
201. Idi1_000022
202. Smp1_000108
203. Ncgc00018099-02
204. Ncgc00018099-03
205. Ncgc00018099-04
206. Ncgc00018099-05
207. Ncgc00018099-06
208. Ncgc00018099-09
209. Ncgc00018099-10
210. Ncgc00089736-02
211. Ncgc00089736-03
212. Ncgc00178894-01
213. Ncgc00178894-02
214. Ac-11186
215. As-12460
216. Cas_479-18-5
217. Sy011592
218. Sbi-0051359.p003
219. Db-051485
220. Ab00051977
221. D3600
222. Ft-0632186
223. Sw196679-3
224. C07819
225. D00691
226. Ab00051977-15
227. Ab00051977_16
228. Ab00051977_17
229. Q-201013
230. Q1073333
231. Sr-01000002972-2
232. Sr-01000002972-3
233. Sr-01000002972-4
234. Brd-a00827783-001-05-5
235. Brd-a00827783-001-15-4
236. Diprophylline, European Pharmacopoeia (ep) Reference Standard
237. Dyphylline, United States Pharmacopeia (usp) Reference Standard
238. 1h-purine-2,6-dione, 7-(2,3-dihydroxypropyl)-3,7-dihydro-1,3-dimethyl-, (+/-)-
| Molecular Weight | 254.24 g/mol |
|---|---|
| Molecular Formula | C10H14N4O4 |
| XLogP3 | -1.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | 254.10150494 g/mol |
| Monoisotopic Mass | 254.10150494 g/mol |
| Topological Polar Surface Area | 98.9 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 364 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Lufyllin |
| PubMed Health | Dyphylline (By mouth) |
| Drug Classes | Bronchodilator |
| Drug Label | LUFYLLIN (dyphylline), a xanthine derivative, is a bronchodilator available for oral administration as tablets containing 200 mg and 400 mg of dyphylline. Other ingredients: magnesium stearate, microcrystalline cellulose.Chemically, dyphylline is 7-(... |
| Active Ingredient | Dyphylline |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 400mg |
| Market Status | Prescription |
| Company | Meda Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Lufyllin |
| PubMed Health | Dyphylline (By mouth) |
| Drug Classes | Bronchodilator |
| Drug Label | LUFYLLIN (dyphylline), a xanthine derivative, is a bronchodilator available for oral administration as tablets containing 200 mg and 400 mg of dyphylline. Other ingredients: magnesium stearate, microcrystalline cellulose.Chemically, dyphylline is 7-(... |
| Active Ingredient | Dyphylline |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 400mg |
| Market Status | Prescription |
| Company | Meda Pharms |
Bronchodilator Agents; Phosphodiesterase Inhibitors; Vasodilator Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
THEOPHYLLINE PREPN...ARE USED TO RELAX BRONCHIAL SMOOTH MUSCLE & TO STIMULATE MYOCARDIUM. ... THEOPHYLLINE COMPD...PLAY IMPORTANT ROLE IN MGMNT OF ASTHMATIC PT. THEY ARE USEFUL AS PROPHYLACTIC DRUGS & ARE VALUABLE ADJUNCTS IN TREATMENT OF PROLONGED ATTACKS & IN MGMNT OF STATUS ASTHMATICUS. /THEOPHYLLINE COMPD/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 375
PERIPHERAL VASODILATOR & BRONCHODILATOR ACTIONS CHARACTERISTIC OF THEOPHYLLINE DERIV. IT IS EFFECTIVE ORALLY BUT HAS NOT BEEN SHOWN TO BE SUPERIOR TO THEOPHYLLINE SODIUM GLYCINATE. IT ALSO HAS TYPICAL DIURETIC & MYOCARDIAL STIMULANT EFFECTS.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1070
...CAN BE USED INTRAMUSCULARLY WITHOUT PRODUCING LOCAL PAIN BECAUSE IT IS NEUTRAL SOL DERIV.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 638
For more Therapeutic Uses (Complete) data for DYPHYLLINE (11 total), please visit the HSDB record page.
IT IS NOT RECOMMENDED FOR USE IN CORONARY DISEASE OR ANGINA PECTORIS UNTIL IT CAN BE SHOWN THAT INCR CORONARY BLOOD FLOW PRECEDES RATHER THAN FOLLOWS MYOCARDIAL STIMULATION.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1070
METHYLXANTHINES CAN ALSO STIMULATE RELEASE OF CATECHOLAMINES FROM ADRENAL MEDULLA & LARGE INCR IN URINARY EXCRETION OF EPINEPHRINE OCCUR... /METHYLXANTHINES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 369
For relief of acute bronchial asthma and for reversible bronchospasm associated with chronic bronchitis and emphysema.
Dyphylline, a xanthine derivative, is a bronchodilator used for relief of acute bronchial asthma and for reversible bronchospasm associated with chronic bronchitis and emphysema. Dyphylline is a xanthine derivative with pharmacologic actions similar to theophylline and other members of this class of drugs. Its primary action is that of bronchodilation, but it also exhibits peripheral vasodilatory and other smooth muscle relaxant activity to a lesser degree.
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Bronchodilator Agents
Agents that cause an increase in the expansion of a bronchus or bronchial tubes. (See all compounds classified as Bronchodilator Agents.)
Phosphodiesterase Inhibitors
Compounds which inhibit or antagonize the biosynthesis or actions of phosphodiesterases. (See all compounds classified as Phosphodiesterase Inhibitors.)
R - Respiratory system
R03 - Drugs for obstructive airway diseases
R03D - Other systemic drugs for obstructive airway diseases
R03DA - Xanthines
R03DA01 - Diprophylline
Route of Elimination
Dyphylline exerts its bronchodilatory effects directly and, unlike theophylline, is excreted unchanged by the kidneys without being metabolized by the liver. Approximately 88% of a single oral dose can be recovered from the urine unchanged.
...RAPID RATE OF DISPOSITION.
Miller, R. R., and D. J. Greenblatt. Handbook of Drug Therapy. New York: Elsevier North Holland, 1979., p. 939
IT MAY BE MORE CONSISTENTLY ABSORBED FROM GI TRACT & LESS IRRITATING THAN THEOPHYLLINE & AMINOPHYLLINE.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 638
SINGLE DYPHYLLINE DOSE WAS GIVEN TO 5 NORMAL VOLUNTEERS. DOSES OF 19-27 MG/KG RESULTED IN PEAK SERUM CONCN OF 19.3-23.5 MUG/ML & WERE TOLERATED WELL BY 4 SUBJECTS. 1 HAD SEVERE HEADACHE AFTER 28 MG/KG DOSE ASSOC WITH 36.4 MUG/ML; WAS NOT METABOLIZED TO THEOPHYLLINE.
PMID:512873 SIMONS KJ, SIMONS FE R; J PHARM SCI 68 (10): 1327 (1979)
IN 5 NORMAL VOLUNTEERS MEAN T/2 WAS 1.8 HR; MEAN TOTAL BODY CLEARANCE RATE & MEAN RENAL CLEARANCE RATE WERE 333 & 276 ML/MIN, RESPECTIVELY. MEAN VOL OF DISTRIBUTION WAS 0.8 L/KG. IN URINE 83% OF DOSE WAS EXCRETED UNCHANGED & THEOPHYLLINE WAS NOT DETECTED.
PMID:512873 SIMONS KJ, SIMONS FE R; J PHARM SCI 68 (10): 1327 (1979)
For more Absorption, Distribution and Excretion (Complete) data for DYPHYLLINE (6 total), please visit the HSDB record page.
Hepatic
IN BODY XANTHINES ARE ONLY PARTIALLY DEMETHYLATED & OXIDIZED. THEY ARE LARGELY EXCRETED AS METHYLURIC ACIDS OR AS METHYLXANTHINES. /XANTHINES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 374
2 hours (range 1.8 - 2.1 hours)
The bronchodilatory action of dyphylline, as with other xanthines, is thought to be mediated through competitive inhibition of phosphodiesterase with a resulting increase in cyclic AMP producing relaxation of bronchial smooth muscle as well as antagonism of adenosine receptors.
THEIR MOST IMPORTANT ACTION...IS THEIR ABILITY TO RELAX SMOOTH MUSCLES OF BRONCHI... THEOPHYLLINE IS MOST EFFECTIVE... /THEOPHYLLINE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 370
ACTION OF XANTHINES ON MOTOR ACTIVITY OF GI TRACT... DILUTE SOLN INCR, & HIGH CONCN DEPRESS, TONE & STRENGTH OF CONTRACTION OF ISOLATED INTESTINAL STRIPS. /XANTHINES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 370
...ACTION SEEMS TO RESULT FROM INCR IN CONCN OF FACTOR V (AC-GLOBULIN) IN PLASMA WHICH, IN TURN, MAY BE CAUSED BY INCR IN PLASMA CONCN OF FREE FATTY ACIDS PRODUCED...THERE ARE ALSO INCR IN CONCN OF CIRCULATING PROTHROMBIN & FIBRINOGEN. /XANTHINES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 371
...ACCOMPANYING DECR IN VENOUS FILLING PRESSURE, WHICH IS CAUSED @ LEAST PARTLY BY MORE COMPLETE EMPTYING OF HEART. /THEOPHYLLINE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 369