
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
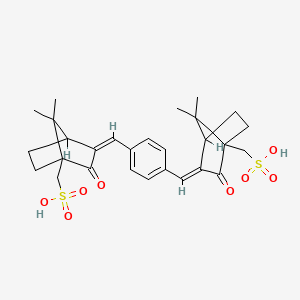

1. 3,3'-(1,4-phenylenedimethylidyne)bis(7,7-dimethyl-2-oxobicyclo(2.2.1)heptane-1-methanesulfonic Acid)
2. Mexoryl Sx
3. Terephthalylidene Dicamphor Sulfonic Acid
1. 90457-82-2
2. 92761-26-7
3. Terephthalylidene Dicamphor Sulfonic Acid
4. Schembl119994
5. Amy215
6. [2-[[4-[[7,7-dimethyl-3-oxo-4-(sulfomethyl)-2-bicyclo[2.2.1]heptanylidene]methyl]phenyl]methylidene]-7,7-dimethyl-3-oxo-4-bicyclo[2.2.1]heptanyl]methanesulfonic Acid
| Molecular Weight | 562.7 g/mol |
|---|---|
| Molecular Formula | C28H34O8S2 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 6 |
| Exact Mass | 562.16951039 g/mol |
| Monoisotopic Mass | 562.16951039 g/mol |
| Topological Polar Surface Area | 160 Ų |
| Heavy Atom Count | 38 |
| Formal Charge | 0 |
| Complexity | 1230 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 4 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Ultraviolet screen
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Cambridge, UK: Royal Society of Chemistry, 2013., p. 639
/EXPL THER/ The aim of this study was to determine, for regulatory purposes, the potential of Mexoryl SX, a broad UVA absorber that also absorbs to some extent in the UVB, to modify the UV radiation (UVR)-induced murine skin tumor development and growth. Skh-hr1 mice were exposed to solar-simulated UVR 5 days per week for 40 weeks. Two control groups were irradiated without topical application, three groups received a sunscreen preparation containing either the UVA absorber, Mexoryl SX at 5 or 10% concentration, or a filter that absorbs principally in the UVB, 2-ethylhexyl-p-methoxycinnamate (2-EHMC) at 5% concentration, introduced as a comparator test article. Sunscreen application was performed before UVR exposure 3 days per week and after UVR exposure on the other 2 days (consistent with the design of a standard photocarcinogenesis safety test). Two different weekly UVR doses were administrated: the lower dose was given to one group of unprotected animals, whereas the higher dose was administrated to the other unprotected group and to the three sunscreen-treated groups. The two UVR control groups demonstrated a UVR-dependent response for cumulative tumor prevalence, tumor yield and median latent period. Neither concentration of Mexoryl SX increased the probability of tumor development; consistent with the principles for safety testing, this provides evidence in that it is safe for use in sunlight. Although this study was explicitly designed as a safety test, the results also provide some clues about the efficacy of Mexoryl SX in decreasing the probability of tumor development. Topical administration of Mexoryl SX, at both concentrations, resulted in a 6 week delay in the median latent period compared to high UVR controls, whereas 5% 2-EHMC delayed the median latent periods only by 2 weeks. Tumor prevalence and yield show the same efficacy differences between the two sunscreen ingredients. Tumor protection factors were calculated from these results and found to be equal to 2.4 for the two preparations containing Mexoryl SX and to 1.3 for the 5% 2-EHMC preparation. These findings illustrate the efficacy of Mexoryl SX in preventing UVR-induced carcinogenesis.
PMID:8863475 Fourtanier A; Photochem Photobiol 64 (4): 688-93 (1996)
/EXPL THER/ In a previous study on the hairless mouse it was shown that sub-erythemal doses of pure UV-A enhanced the numerous changes normally observed during chronological aging. A new sunscreen (a bis-benzylidene campho sulfonic acid derivative) has been synthesized in our research laboratory (lambda max: 345 nm, epsilon: 47,000). Its photoprotective properties against UV-A induced damages were assessed in our mouse model. Three-month-old albino hairless mice were exposed for 1 y to suberythemal doses (35 J/sq cm) of UV-A obtained from a xenon source filtered through a WG 345 filter. One group of animals was exposed untreated, the other received a formulation containing 5% of the sunscreen prior to irradiation. At the end of the study the cutaneous properties of protected mice were compared to those of unprotected animals and to 3 and 15-month-old unirradiated controls. We found that the visible changes induced by UV-A irradiation were mainly sagging and wrinkling. Histological and electron microscopic alterations consisted of hyperkeratosis, increased density of elastic fibers with alteration of fiber orientation and increased glycosaminoglycan deposits. Biochemical changes consisted of decreases in total collagen and collagen hydroxylation and increases in both collagen III/I + III ratio and fibronectin biosynthesis. All these changes were reduced or abolished by the sunscreen.
PMID:1320278 Fourtanier A et al; Photochem Photobiol 55 (4): 549-60 (1992)
/EXPL THER/ BACKGROUND/PURPOSE: Exposure to ultraviolet (UV) radiation increases skin pigmentation and usually results in an even darkening of the skin. However, it may also occasionally lead to the development of hyperpigmented lesions due to a local overproduction of pigment. Skin pigmentation is induced both by UVB and UVA rays. METHODS: The in vivo protection by sunscreens against pigmentation was studied using the determination of a level of protection against pigmentation based on the standardized sun protection factor (SPF) test method. The method includes delayed UVB and UVA pigmentations. The level of prevention against pigmentation was determined 7 days after exposure to solar-simulated radiation by visual assessment. It was calculated using the ratio of the minimal pigmenting dose on protected skin to the minimal pigmenting dose on unprotected skin. Broadspectrum UVB/UVA filters, Mexoryl SX and Mexoryl XL, and complete formula were tested. RESULTS: Protection against pigmentation correlates with the concentration of Mexoryl SX. The levels of protection obtained show a synergetic effect of Mexoryl SX when associated with Mexoryl XL. When different products having the same SPF (same protection against erythema) and different levels of UVA protection are compared, only sunscreen products with a high level of UVA protection show a similar level of protection against sunburn and pigmentation. Products with low UVA protection have a lower capacity of preventing induced pigmentation compared with their efficacy against erythema. CONCLUSIONS: These studies have evidenced that SPF determination was not sufficient to account for the efficiency in preventing pigmentation and that UVA protection was an essential part of this prevention.
PMID:15379874 Moyal D; Photodermatol Photoimmunol Photomed 20 (5): 243-7 (2004)
For more Therapeutic Uses (Complete) data for Terephthalylidene dicamphor sulfonic acid (6 total), please visit the HSDB record page.
Sunscreening Agents
Chemical or physical agents that protect the skin from sunburn and erythema by absorbing or blocking ultraviolet radiation. (See all compounds classified as Sunscreening Agents.)
The potential human health risk of UV filters depends on their toxicity and the human systemic exposure which is a function of the extent of percutaneous absorption of the topically applied substance into the human organism. Using a 'mass balance' approach, a study was designed to investigate the systemically absorbed dose of [(14)C]-Mexoryl SX((R)) in humans after topical application of a typical sunscreen emulsion. In addition, to assess the correlation with in vitro experiments, the percutaneous absorption of this UVA filter through isolated human skin was measured under identical exposure conditions. When applied in vivo for a period of 4 hr, 89-94% of the applied radioactivity was recovered from the wash-off samples. In urine samples, the radioactivity slightly exceeded background levels and corresponded maximally to 0.014% of the topically applied dose. No radioactivity was measured in blood or feces sampled up to 120 hr after application. In vitro, 24 hr after a 4-hour application, [(14)C]-Mexoryl SX remained primarily on the skin surface. The mean in vitro absorption over 24 hr, adding up the amounts found in the dermis and receptor fluid, was 0.16% of the applied dose. It is concluded from the in vivo pharmacokinetic results that the systemically absorbed dose of [(14)C]-Mexoryl SX is less than 0.1%. The order of magnitude of this value correlates well with the corresponding in vitro data which overestimate the in vivo results as previously observed with other hydrophilic compounds. This study demonstrates that, under realistic exposure conditions, the human systemic exposure to this UVA filter is negligible and poses no risk to human health.
PMID:14528058 Benech-Kieffer F et al; Skin Pharmacol Appl Skin Physiol 16 (6): 343-55 (2003)
An in vivo method in humans using radioactive terephthalylidene dicamphor sulfonic acid ((14)C site not specified) showed an absorption determined up to 24 hours after a 4-hours exposure to 0.16% of the applied dose.
The Danish Environmental Protection Agency. Survey and health assessment of UV filters. p.170 (2015)
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
56
PharmaCompass offers a list of Ecamsule API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ecamsule manufacturer or Ecamsule supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ecamsule manufacturer or Ecamsule supplier.
PharmaCompass also assists you with knowing the Ecamsule API Price utilized in the formulation of products. Ecamsule API Price is not always fixed or binding as the Ecamsule Price is obtained through a variety of data sources. The Ecamsule Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ecamsule manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ecamsule, including repackagers and relabelers. The FDA regulates Ecamsule manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ecamsule API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ecamsule manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ecamsule supplier is an individual or a company that provides Ecamsule active pharmaceutical ingredient (API) or Ecamsule finished formulations upon request. The Ecamsule suppliers may include Ecamsule API manufacturers, exporters, distributors and traders.
click here to find a list of Ecamsule suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Ecamsule Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ecamsule GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ecamsule GMP manufacturer or Ecamsule GMP API supplier for your needs.
A Ecamsule CoA (Certificate of Analysis) is a formal document that attests to Ecamsule's compliance with Ecamsule specifications and serves as a tool for batch-level quality control.
Ecamsule CoA mostly includes findings from lab analyses of a specific batch. For each Ecamsule CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ecamsule may be tested according to a variety of international standards, such as European Pharmacopoeia (Ecamsule EP), Ecamsule JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ecamsule USP).
