
Synopsis
Synopsis
0
CEP/COS
0
KDMF
0
VMF
0
Europe

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
Regulatory FDF Prices
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
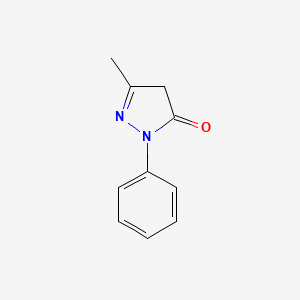

1. 1 Phenyl 3 Methyl 5 Pyrazolone
2. 1-phenyl-3-methyl-5-pyrazolone
3. 3 Methyl 1 Phenyl 2 Pyrazolin 5 One
4. 3-methyl-1-phenyl-2-pyrazolin-5-one
5. Edarabone
6. Mci 186
7. Mci-186
8. Mci186
9. Norantipyrine
10. Norphenazone
11. Phenylmethylpyrazolone
12. Radicava
1. 89-25-8
2. 3-methyl-1-phenyl-2-pyrazolin-5-one
3. Radicut
4. Norphenazone
5. Mci-186
6. Developer Z
7. Methylphenylpyrazolone
8. Norantipyrine
9. C.i. Developer 1
10. Phenyl Methyl Pyrazolone
11. Phenylmethylpyrazolone
12. Radicava
13. 3-methyl-1-phenyl-1h-pyrazol-5(4h)-one
14. 5-methyl-2-phenyl-2,4-dihydro-3h-pyrazol-3-one
15. 1-phenyl-3-methyl-5-oxo-2-pyrazoline
16. 5-methyl-2-phenyl-4h-pyrazol-3-one
17. 1-phenyl-3-methylpyrazolone
18. 3h-pyrazol-3-one, 2,4-dihydro-5-methyl-2-phenyl-
19. 1-phenyl-3-methylpyrazolone-5
20. 3-methyl-1-phenylpyrazol-5-one
21. 3-methyl-1-phenyl-2-pyrazoline-5-one
22. Nci-c03952
23. 2-pyrazolin-5-one, 3-methyl-1-phenyl-
24. 5-pyrazolone, 3-methyl-1-phenyl-
25. 2,4-dihydro-5-methyl-2-phenyl-3h-pyrazol-3-one
26. Edaravone (mci-186)
27. 1-fenyl-3-methyl-2-pyrazolin-5-on
28. 3-methyl-1-phenyl-4,5-dihydro-1h-pyrazol-5-one
29. Chebi:31530
30. Nsc-2629
31. Nsc-26139
32. Antipyrine Related Compound A
33. Mls000069602
34. 3-methyl-1-phenyl-2-pyrazolin-5-one (mci-186)
35. Ci Developer 1
36. Ncgc00164015-01
37. Smr000059020
38. Dsstox_cid_1130
39. Dsstox_rid_75961
40. Dsstox_gsid_21130
41. Monopyrazolone
42. Wln: T5nmv Dhj Br& E1
43. Cas-89-25-8
44. Ccris 512
45. Radicut (tn)
46. Hsdb 4102
47. 3h-pyrazol-3-one,4-dihydro-5-methyl-2-phenyl-
48. Sr-01000000135
49. 1-fenyl-3-methyl-2-pyrazolin-5-on [czech]
50. Einecs 201-891-0
51. Mfcd00003138
52. Unii-s798v6yjrp
53. Brn 0609575
54. Ai3-03557
55. Mci186
56. (edaravone)
57. Radicava (tn)
58. (mci-186)
59. Cds1_000986
60. Spectrum_000267
61. Tocris-0786
62. Mci-186; Edaravone
63. Edaravone [usan:inn]
64. Maybridge1_005738
65. Opera_id_1057
66. Spectrum2_001574
67. Spectrum3_000971
68. Spectrum4_001091
69. Spectrum5_001217
70. M0687
71. Ec 201-891-0
72. Schembl4704
73. Bspbio_001235
74. Bspbio_002601
75. Kbiogr_000575
76. Kbiogr_001502
77. Kbioss_000575
78. Kbioss_000747
79. Ae-641/00371017
80. Mls001146878
81. Mls002415675
82. Mls006011753
83. Divk1c_001018
84. Divk1c_002026
85. Spectrum1503635
86. Spbio_001508
87. Chembl290916
88. Edaravone (usan/jp17/inn)
89. Dtxsid9021130
90. Bcbcmap01_000127
91. Gtpl11994
92. Hms503k17
93. Hms557m18
94. Kbio1_001018
95. Kbio2_000575
96. Kbio2_000747
97. Kbio2_003143
98. Kbio2_003315
99. Kbio2_005711
100. Kbio2_005883
101. Kbio3_001029
102. Kbio3_001030
103. Kbio3_001821
104. Nsc2629
105. Ninds_001018
106. Bcpp000246
107. Bio1_000438
108. Bio1_000927
109. Bio1_001416
110. Bio2_000448
111. Bio2_000928
112. Hms1362m17
113. Hms1792m17
114. Hms1990m17
115. Hms2234m19
116. Hms3266f04
117. Hms3403m17
118. Hms3411l05
119. Hms3654l15
120. Hms3675l05
121. Hms3884a11
122. Pharmakon1600-01503635
123. Act07289
124. Bcp26336
125. Hy-b0099
126. Nsc26139
127. Tox21_112077
128. Tox21_201747
129. Tox21_302819
130. Bdbm50200541
131. Ccg-39352
132. Nsc758622
133. S1326
134. Stk201315
135. Zinc18203737
136. 3-methyl-1-phenyl-2-pyrazolin-5one
137. Akos000313817
138. Tox21_112077_1
139. Ac-4745
140. Bcp9000635
141. Cs-1832
142. Db12243
143. Nsc-758622
144. Sb19128
145. Idi1_001018
146. Idi1_002203
147. 1-pehnyl-3-methyl-5-pyrazalone
148. Ncgc00018218-01
149. Ncgc00018218-02
150. Ncgc00018218-03
151. Ncgc00018218-04
152. Ncgc00018218-05
153. Ncgc00018218-06
154. Ncgc00018218-07
155. Ncgc00018218-08
156. Ncgc00018218-10
157. Ncgc00018218-17
158. Ncgc00022665-02
159. Ncgc00022665-04
160. Ncgc00022665-05
161. Ncgc00022665-06
162. Ncgc00256515-01
163. Ncgc00259296-01
164. Sbi-0051836.p002
165. Db-002517
166. Am20060748
167. Ft-0608243
168. Sw148216-2
169. 5-methyl-2-phenyl-2,4-dihydro-3-pyrazolone
170. 3-methyl-1-phenyl-2-pyrazoline-5-one, 99%
171. 4e-901
172. 5-methyl-2-phenyl-2,4-dihydro-pyrazol-3-one
173. D01552
174. D86209
175. 3-?methyl-?1-?phenyl-?2-?pyrazolin-?5-?one
176. Ab00375776_14
177. Ab00375776_15
178. 2 4-dihydro-5-methyl-2-phenyl-3h-pyrazol-3-one
179. 2,4-dihydro-2-phenyl-5-methyl-3h-pyrazol-3-one
180. A843105
181. Q335099
182. Q-200386
183. Sr-01000000135-2
184. Sr-01000000135-3
185. Sr-01000000135-5
186. 5-methyl-2-phenyl-2,4-dihydro-3h-pyrazol-3-one #
187. Brd-k35458079-001-04-2
188. Brd-k35458079-001-12-5
189. Brd-k35458079-001-23-2
190. Z50145861
191. F0391-0021
192. 3-methyl-1-phenyl-2-pyrazoline-5-one, Saj Special Grade
193. 3-methyl-1-phenyl-2-pyrazoline-5-one, Purum, >=98.0% (nt)
194. 5-methyl-2-phenyl-2,4-dihydro-3h-pyrazol-3-one (edaravone)
195. Phenazone Impurity A, European Pharmacopoeia (ep) Reference Standard
196. 5-methyl-2-phenyl-1,2-dihydropyrazol-3-one;3-methyl-1-phenyl-2-pyrazolin-5-one
197. Antipyrine Related Compound A, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 174.20 g/mol |
|---|---|
| Molecular Formula | C10H10N2O |
| XLogP3 | 1.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 174.079312947 g/mol |
| Monoisotopic Mass | 174.079312947 g/mol |
| Topological Polar Surface Area | 32.7 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 241 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated for improving neurological symptoms and damage from acute ischemic stroke and delaying disease progression of ALS.
FDA Label
Treatment of amyotrophic lateral sclerosis
Treatment of amyotrophic lateral sclerosis
Edaravone scavenges free hydroxyl radicals and peroxynitrite radicals which are highly associated with neuronal damage/death from many cerebral vascular disorders such as ischemic strokes and degenerative neurological disorders such as ALS. It exerts a neuroprotective and antioxidant effect and delays disease progression by limiting the extent of lipid peroxidation via free radical generation and cell membrane damage from oxidative stress. It reversed the reduction in regional blood flow and cerebral edema in a case of ischemic stroke.
Free Radical Scavengers
Substances that eliminate free radicals. Among other effects, they protect PANCREATIC ISLETS against damage by CYTOKINES and prevent myocardial and pulmonary REPERFUSION INJURY. (See all compounds classified as Free Radical Scavengers.)
Neuroprotective Agents
Drugs intended to prevent damage to the brain or spinal cord from ischemia, stroke, convulsions, or trauma. Some must be administered before the event, but others may be effective for some time after. They act by a variety of mechanisms, but often directly or indirectly minimize the damage produced by endogenous excitatory amino acids. (See all compounds classified as Neuroprotective Agents.)
N - Nervous system
N07 - Other nervous system drugs
N07X - Other nervous system drugs
N07XX - Other nervous system drugs
N07XX14 - Edaravone
Absorption
The peak plasma concentration of the parent drug is reached at the end of infusion, without accumulation of the drug with multiple dosing regimen. The mean Cmax value in healthy male adults is 888ng/mL for intravenous infusion. The values of AUC and Cmax are increased in a dose-proportional relationship. The oral bioavailability in mouse studies is 38% of the I.V. delivery.
Route of Elimination
About 0.7-0.9% of the dose is excreted as unchanged drug and 71.0-79.9% of the dose is excreted as metabolites (mostly as glucuronide conjugates) through mainly renal elimination.
Volume of Distribution
The mean Vd value following an intravenous infusion of a single 30mg dose is 18.5L/kg.
Clearance
The mean total plasma drug clearance following an intravenous infusion of a single 30mg dose is 0.1L/min.
Multiple renal and hepatic uridine diphosphate glucuronosyltransferase (UGT) isoforms catalyze glucuronidation reaction of edaravone to form glucuronide conjugates. Edaravone is also metabolized into sulfate conjugates via sulfotransferase activity, which is the main metabolite form predominantly found circulating in plasma. It is predicted that the sulfate conjugate is hydrolyzed back to edaravone, which is then converted to the glucuronide conjugate in the human kidney before excretion into the urine. These metabolites have no pharmacological activity.
The mean terminal elimination half-life of edaravone is 4.5 to 6 hours and the half-lives of its metabolites are 2 to 2.8 hours.
Nootropic and neuroprotective effects are mediated through inhibiting lipid peroxidation and scavenging free radicals. Edaravone acts to increase prostacyclin production, decrease lipoxygenase metabolism of arachidonic acid by trapping hydroxyl radicals, and inhibit alloxan-induced lipid peroxidation and quench active oxygen species. It targets various kinds of cells, including neurons, endothelial cells and myocardial cells. There is also evidence of reduction of neuronal nitric oxide synthase (nNOS) levels and potentiation of SOD1 levels after transient ischemia in rabbits thus preventing spinal cord injury.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
45
PharmaCompass offers a list of Edaravone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Edaravone manufacturer or Edaravone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Edaravone manufacturer or Edaravone supplier.
PharmaCompass also assists you with knowing the Edaravone API Price utilized in the formulation of products. Edaravone API Price is not always fixed or binding as the Edaravone Price is obtained through a variety of data sources. The Edaravone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Edaravone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Edaravone, including repackagers and relabelers. The FDA regulates Edaravone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Edaravone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Edaravone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Edaravone supplier is an individual or a company that provides Edaravone active pharmaceutical ingredient (API) or Edaravone finished formulations upon request. The Edaravone suppliers may include Edaravone API manufacturers, exporters, distributors and traders.
click here to find a list of Edaravone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Edaravone DMF (Drug Master File) is a document detailing the whole manufacturing process of Edaravone active pharmaceutical ingredient (API) in detail. Different forms of Edaravone DMFs exist exist since differing nations have different regulations, such as Edaravone USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Edaravone DMF submitted to regulatory agencies in the US is known as a USDMF. Edaravone USDMF includes data on Edaravone's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Edaravone USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Edaravone suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Edaravone Drug Master File in Japan (Edaravone JDMF) empowers Edaravone API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Edaravone JDMF during the approval evaluation for pharmaceutical products. At the time of Edaravone JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Edaravone suppliers with JDMF on PharmaCompass.
A Edaravone written confirmation (Edaravone WC) is an official document issued by a regulatory agency to a Edaravone manufacturer, verifying that the manufacturing facility of a Edaravone active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Edaravone APIs or Edaravone finished pharmaceutical products to another nation, regulatory agencies frequently require a Edaravone WC (written confirmation) as part of the regulatory process.
click here to find a list of Edaravone suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Edaravone as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Edaravone API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Edaravone as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Edaravone and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Edaravone NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Edaravone suppliers with NDC on PharmaCompass.
Edaravone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Edaravone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Edaravone GMP manufacturer or Edaravone GMP API supplier for your needs.
A Edaravone CoA (Certificate of Analysis) is a formal document that attests to Edaravone's compliance with Edaravone specifications and serves as a tool for batch-level quality control.
Edaravone CoA mostly includes findings from lab analyses of a specific batch. For each Edaravone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Edaravone may be tested according to a variety of international standards, such as European Pharmacopoeia (Edaravone EP), Edaravone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Edaravone USP).
