
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Du-176
2. Du-176b
3. Edoxaban Tosylate
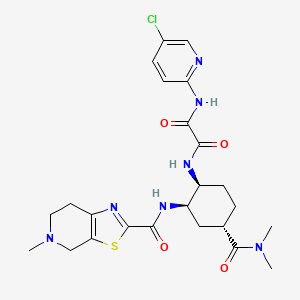
4. N-(5-chloropyridin-2-yl)-n'-((1s,2r,4s)-4-(n,n-dimethylcarbamoyl)-2-(5-methyl-4,5,6,7- Tetrahydro(1,3)thiazolo(5,4-c)pyridine-2-carboxamido)cyclohexyl)oxamide
5. N-(5-chloropyridin-2-yl)-n'-((1s,2r,4s)-4-(n,n-dimethylcarbamoyl)-2-(5-methyl-4,5,6,7-tetrahydrothiazolo(5,4-c)pyridine-2-carboxamido)cyclohexyl)ethanediamide P-toluenesulfonate Monohydrate
6. Savaysa
1. 480449-70-5
2. 912273-65-5
3. Du-176
4. Du-176b
5. Ndu3j18apo
6. N1-(5-chloropyridin-2-yl)-n2-((1s,2r,4s)-4-(dimethylcarbamoyl)-2-(5-methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridine-2-carboxamido)cyclohexyl)oxalamide
7. Chembl1269025
8. Chebi:85973
9. 480449-70-5 (free Base)
10. N-(5-chloropyridin-2-yl)-n'-((1s,2r,4s)-4-(n,n-dimethylcarbamoyl)-2-(5-methyl-4,5,6,7- Tetrahydro(1,3)thiazolo(5,4-c)pyridine-2-carboxamido)cyclohexyl)oxamide
11. Edoxaban [usan]
12. Ethanediamide, N1-(5-chloro-2-pyridinyl)-n2-((1s,2r,4s)-4- ((dimethylamino)carbonyl)- 2-(((4,5,6,7-tetrahydro-5-methylthiazolo(5,4-c)pyridin-2-yl)carbonyl)amino)cyclohexyl)-
13. Edoxaban [usan:inn]
14. Unii-ndu3j18apo
15. Edoxaban (usan/inn)
16. Edoxaban [inn]
17. Edoxaban [mi]
18. Edoxaban [mart.]
19. Edoxaban [who-dd]
20. Schembl330046
21. Amy508
22. Gtpl7575
23. Hsdb 8406
24. Dtxsid50197398
25. Ex-a5582
26. Bdbm50328731
27. Mfcd13195544
28. S4429
29. Zinc43200832
30. Akos005146069
31. Cs-1331
32. Db09075
33. Ncgc00378907-01
34. Ncgc00378907-02
35. Ncgc00378907-03
36. Ac-35419
37. As-35107
38. Hy-10264
39. N-(5-chloro-2-pyridinyl)-n'-[(1s,2r,4s)-4-[(dimethylamino)carbonyl]-2-[[(4,5,6,7-tetrahydro-5-methylthiazolo[5,4-c]pyridin-2-yl)carbonyl]amino]cyclohexyl]ethanediamide
40. D09710
41. 449e705
42. A860576
43. Q21011234
44. N'-(5-chloropyridin-2-yl)-n-[(1s,2r,4s)-4-(dimethylcarbamoyl)-2-[(5-methyl-6,7-dihydro-4h-[1,3]thiazolo[5,4-c]pyridine-2-carbonyl)amino]cyclohexyl]oxamide
45. N'-(5-chloropyridin-2-yl)-n-[(1s,2r,4s)-4-(dimethylcarbamoyl)-2-[(5-methyl6,7-dihydro-4h-[1,3]thiazolo[5,4-c]pyridine-2-carbonyl)amino]cyclohexyl]oxamide
46. N(1)-(5-chloropyridin-2-yl)-n(2)-{(1s,2r,4s)-4-(dimethylcarbamoyl)-2-[(5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carbonyl)amino]cyclohexyl}ethanediamide
47. N-(5-chloro-2-pyridinyl)-n'-[4-(dimethylcarbamoyl)-2-{[(5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridin-2-yl)carbonyl]amino}cyclohexyl]ethanediamide;
48. N-(5-chloropyridin-2-yl)-n'-[(1s,2r,4s)-4-(dimethylcarbamoyl)-2-[(5-methyl-6,7-dihydro-4h-[1,3]thiazolo[5,4-c]pyridine-2-carbonyl)amino]cyclohexyl]oxamide
49. N-(5-chloropyridin-2-yl)-n'-[(1s,2r,4s)-4-(dimethylcarbamoyl)-2-{[(5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridin-2-yl)carbonyl]amino}cyclohexyl]ethanediamide
50. N1-(5-chloropyridin-2-yl)-n2-((1s,2r,4s)-4-[(dimethylamino)carbonyl]-2-{[(5-methyl-4,5, 6,7-tetrahydrothiazolo[5,4-c]pyridin-2-yl)carbonyl]amino}cyclohexyl) Ethanediamide
51. N1-(5-chloropyridin-2-yl)-n2-((1s,2r,4s)-4-[(dimethylamino)carbonyl]-2-{[(5-methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridin-2-yl)carbonyl]amino}cyclohexyl)ethanediamide
52. N1-(5-chloropyridin-2-yl)-n2-[(1s,2r,4s)-4-(dimethylcarbamoyl)-2-{[(5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridin-2-yl)carbonyl]amino}cyclohexyl]ethanediamide
1. Edoxaban (tosylate)
2. Edoxaban Tsoh Salt
| Molecular Weight | 548.1 g/mol |
|---|---|
| Molecular Formula | C24H30ClN7O4S |
| XLogP3 | 1.4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 547.1768513 g/mol |
| Monoisotopic Mass | 547.1768513 g/mol |
| Topological Polar Surface Area | 165 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 880 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Factor Xa Inhibitors
National Library of Medicine's Medical Subject Headings. Edoxaban. Online file (MeSH, 2018). Available from, as of March 7, 2018: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Edoxaban is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of March 7, 2018: https://clinicaltrials.gov/
Savaysa is indicated to reduce the risk of stroke and systemic embolism (SE) in patients with nonvalvular atrial fibrillation (NVAF). /Included in US product label/
NIH; DailyMed. Current Medication Information for Savaysa (Edoxaban Tosylate) Tablet, Film-coated (Updated: November 6, 2017). Available from, as of March 22, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e77d3400-56ad-11e3-949a-0800200c9a66
Savaysa is indicated for the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) following 5 to 10 days of initial therapy with a parenteral anticoagulant. /Included in US product label/
NIH; DailyMed. Current Medication Information for Savaysa (Edoxaban Tosylate) Tablet, Film-coated (Updated: November 6, 2017). Available from, as of March 22, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e77d3400-56ad-11e3-949a-0800200c9a66
For more Therapeutic Uses (Complete) data for Edoxaban (7 total), please visit the HSDB record page.
/BOXED WARNING/ REDUCED EFFICACY IN NONVALVULAR ATRIAL FIBRILLATION PATIENTS WITH CRCL > 95 ML/MIN. Savaysa should not be used in patients with CrCL > 95 mL/min. In the ENGAGE AF-TIMI 48 study, nonvalvular atrial fibrillation patients with CrCL > 95 mL/min had an increased rate of ischemic stroke with Savaysa 60 mg once daily compared to patients treated with warfarin. In these patients another anticoagulant should be used.
NIH; DailyMed. Current Medication Information for Savaysa (Edoxaban Tosylate) Tablet, Film-coated (Updated: November 6, 2017). Available from, as of March 22, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e77d3400-56ad-11e3-949a-0800200c9a66
/BOXED WARNING/ PREMATURE DISCONTINUATION OF SAVAYSA INCREASES THE RISK OF ISCHEMIC EVENTS. Premature discontinuation of any oral anticoagulant in the absence of adequate alternative anticoagulation increases the risk of ischemic events. If Savaysa is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant as described in the transition guidance.
NIH; DailyMed. Current Medication Information for Savaysa (Edoxaban Tosylate) Tablet, Film-coated (Updated: November 6, 2017). Available from, as of March 22, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e77d3400-56ad-11e3-949a-0800200c9a66
/BOXED WARNING/ SPINAL/EPIDURAL HEMATOMA. Epidural or spinal hematomas may occur in patients treated with Savaysa who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include: use of indwelling epidural catheters; concomitant use of other drugs that affect hemostasis, such as nonsteroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, other anticoagulants; a history of traumatic or repeated epidural or spinal punctures; a history of spinal deformity or spinal surgery; optimal timing between the administration of Savaysa and neuraxial procedures is not known. Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessar. Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated.
NIH; DailyMed. Current Medication Information for Savaysa (Edoxaban Tosylate) Tablet, Film-coated (Updated: November 6, 2017). Available from, as of March 22, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e77d3400-56ad-11e3-949a-0800200c9a66
Safety and efficacy of edoxaban have not been evaluated in patients with mechanical heart valves or moderate to severe mitral stenosis; use of the drug is not recommended in such patients.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 1639
For more Drug Warnings (Complete) data for Edoxaban (18 total), please visit the HSDB record page.
Edoxaban is indicated for reducing the risk of stroke and systemic embolism (SE) in patients with nonvalvular atrial fibrillation (NVAF). However, it should not be used in patients with creatinine clearance (CrCL) > 95 mL/min because of increased risk of ischemic stroke compared to warfarin at the highest dose studied (60 mg). It is also indicated for the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) following 5-10 days of initial therapy with a parenteral anticoagulant.
FDA Label
Prevention of stroke and systemic embolism in adult patients with nonvalvular atrial fibrillation (NVAF) with one or more risk factors, such as congestive heart failure, hypertension, age 75 years, diabetes mellitus, prior stroke or transient ischaemic attack (TIA).
Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults.
Prevention of stroke and systemic embolism in adult patients with nonvalvular atrial fibrillation (NVAF) with one or more risk factors, such as congestive heart failure, hypertension, age 75 years, diabetes mellitus, prior stroke or transient ischaemic attack (TIA).
Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults.
Administration of edoxaban results in prolongation of clotting time tests such as aPTT (activated partial thromboplastin time), PT (prothrombin time), and INR (international normalized ratio).
Factor Xa Inhibitors
Endogenous factors and drugs that inhibit or block the activity of FACTOR XA. (See all compounds classified as Factor Xa Inhibitors.)
B01
B01AF03
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AF - Direct factor xa inhibitors
B01AF03 - Edoxaban
Absorption
Following oral administration, peak plasma edoxaban concentrations are observed within 1-2 hours. Absolute bioavailability is 62%.
Route of Elimination
Edoxaban is eliminated primarily as unchanged drug in urine. Renal clearance (11 L/hour) accounts for approximately 50% of the total clearance of edoxaban (22 L/hour). Metabolism and biliary/intestinal excretion account for the remaining clearance.
Volume of Distribution
The steady state volume of distribution is 107 L.
Clearance
22 L/hr
/MILK/ There are no data on the presence of edoxaban in human milk ... . Edoxaban was present in rat milk. ...
NIH; DailyMed. Current Medication Information for Savaysa (Edoxaban Tosylate) Tablet, Film-coated (Updated: November 6, 2017). Available from, as of March 22, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e77d3400-56ad-11e3-949a-0800200c9a66
Disposition is biphasic. The steady-state volume of distribution (Vdss) is 107 (19.9) L (mean (SD)). In vitro plasma protein binding is approximately 55%. There is no clinically relevant accumulation of edoxaban (accumulation ratio 1.14) with once daily dosing.
NIH; DailyMed. Current Medication Information for Savaysa (Edoxaban Tosylate) Tablet, Film-coated (Updated: November 6, 2017). Available from, as of March 22, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e77d3400-56ad-11e3-949a-0800200c9a66
Administration of a crushed 60 mg tablet, either mixed into applesauce or suspended in water and given through a nasogastric tube, showed similar exposure compared to administration of an intact tablet.
NIH; DailyMed. Current Medication Information for Savaysa (Edoxaban Tosylate) Tablet, Film-coated (Updated: November 6, 2017). Available from, as of March 22, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e77d3400-56ad-11e3-949a-0800200c9a66
Edoxaban is eliminated primarily as unchanged drug in the urine. Renal clearance (11 L/hour) accounts for approximately 50% of the total clearance of edoxaban (22 L/hour). Metabolism and biliary/intestinal excretion account for the remaining clearance.
NIH; DailyMed. Current Medication Information for Savaysa (Edoxaban Tosylate) Tablet, Film-coated (Updated: November 6, 2017). Available from, as of March 22, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e77d3400-56ad-11e3-949a-0800200c9a66
Following oral administration, peak plasma edoxaban concentrations are observed within 1-2 hours. Absolute bioavailability is 62%. Food does not affect total systemic exposure to edoxaban. Savaysa was administered with or without food in the ENGAGE AF-TIMI 48 and Hokusai VTE trials.
NIH; DailyMed. Current Medication Information for Savaysa (Edoxaban Tosylate) Tablet, Film-coated (Updated: November 6, 2017). Available from, as of March 22, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e77d3400-56ad-11e3-949a-0800200c9a66
Edoxaban is not extensively metabolized by CYP3A4 resulting in minimal drug-drug interactions. However, it does interact with drugs that inhibit p-gp (p-glycoprotein), which is used to transport edoxaban across the intestinal wall. Unchanged edoxaban is the predominant form in plasma. There is minimal metabolism via hydrolysis (mediated by carboxylesterase 1), conjugation, and oxidation by CYP3A4. The predominant metabolite M-4, formed by hydrolysis, is human-specific and active and reaches less than 10% of the exposure of the parent compound in healthy subjects. Exposure to the other metabolites is less than 5% of exposure to edoxaban.
... All subjects received a single oral 60 mg edoxaban dose in period 1, and 7 days of 600 mg rifampin (2 x 300 mg capsules once daily) with a single oral edoxaban 60 mg dose administered concomitantly on day 7 in period 2. A 6-day washout period separated the treatments. Plasma concentrations of edoxaban and its metabolites M4 and M6 were measured, and limited assessments of pharmacodynamic markers of coagulation were performed. In total, 34 healthy subjects were enrolled; 32 completed the study. Coadministration of rifampin with edoxaban decreased edoxaban exposure but increased active metabolite exposure. Rifampin increased apparent oral clearance of edoxaban by 33% and decreased its half-life by 50%. Anticoagulant effects based on the prothrombin time (PT) and the activated partial thromboplastin time (aPTT) with and without rifampin at early time points were maintained to a greater-than-expected degree than with edoxaban exposure alone, presumably because of an increased contribution from the active metabolites. Edoxaban was well tolerated in this healthy adult population. Rifampin reduced exposure to edoxaban while increasing exposure to its active metabolites M4 and M6. PT and aPTT at early time points did not change appreciably; however, the data should be interpreted with caution.
PMID:26068927 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4488474 Mendell J et al; Clin Drug Investig 35 (7): 447-53 (2015)
Edoxaban and its low-abundance, active metabolite M4 are substrates of P-glycoprotein (P-gp; MDR1) and organic anion transporter protein 1B1 (OATP1B1), respectively, and pharmacological inhibitors of P-gp and OATP1B1 can affect edoxaban and M4 pharmacokinetics (PK). In this integrated pharmacogenomic analysis, genotype and concentration-time data from 458 healthy volunteers in 14 completed phase 1 studies were pooled to examine the impact on edoxaban PK parameters of allelic variants of ABCB1 (rs1045642: C3435T) and SLCO1B1 (rs4149056: T521C), which encode for P-gp and OATP1B1. Although some pharmacologic inhibitors of P-gp and OATP1B1 increase edoxaban exposure, neither the ABCB1 C3435T nor the SLCO1B1 T521C polymorphism affected edoxaban PK. A slight elevation in M4 exposure was observed among SLCO1B1 C-allele carriers; however, this elevation is unlikely to be clinically significant as plasma M4 concentrations comprise <10% of total edoxaban levels.
PMID:27897269 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5817390 Vandell AG et al; Pharmacogenomics J 18 (1): 153-159 (2018)
The predominant metabolite M-4, formed by hydrolysis, is human-specific and active and reaches less than 10% of the exposure of the parent compound in healthy subjects. Exposure to the other metabolites is less than 5% of exposure to edoxaban.
NIH; DailyMed. Current Medication Information for Savaysa (Edoxaban Tosylate) Tablet, Film-coated (Updated: November 6, 2017). Available from, as of March 22, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e77d3400-56ad-11e3-949a-0800200c9a66
Unchanged edoxaban is the predominant form in plasma. There is minimal metabolism via hydrolysis (mediated by carboxylesterase 1), conjugation, and oxidation by CYP3A4.
NIH; DailyMed. Current Medication Information for Savaysa (Edoxaban Tosylate) Tablet, Film-coated (Updated: November 6, 2017). Available from, as of March 22, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e77d3400-56ad-11e3-949a-0800200c9a66
The terminal elimination half-life of edoxaban following oral administration is 10 to 14 hours.
The terminal elimination half-life of edoxaban following oral administration is 10 to 14 hours.
NIH; DailyMed. Current Medication Information for Savaysa (Edoxaban Tosylate) Tablet, Film-coated (Updated: November 6, 2017). Available from, as of March 22, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e77d3400-56ad-11e3-949a-0800200c9a66
Edoxaban is a selective inhibitor of factor Xa, a serine endopeptidase of the clotting cascade required for cleavage of prothrombin into thrombin.
Edoxaban tosylate monohydrate, an oral, direct activated factor X (Xa) inhibitor, is an anticoagulant. Factor Xa plays a central role in the blood coagulation cascade by serving as the convergence point for the intrinsic and extrinsic pathways; inhibition of coagulation factor Xa by edoxaban prevents conversion of prothrombin to thrombin and subsequent thrombus formation. The drug binds directly and selectively to factor Xa without the need for a cofactor (e.g., antithrombin III), and inhibits both free and prothrombinase-bound factor Xa as well as thrombin-induced platelet aggregation.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 1640

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
61
PharmaCompass offers a list of Edoxaban API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Edoxaban manufacturer or Edoxaban supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Edoxaban manufacturer or Edoxaban supplier.
PharmaCompass also assists you with knowing the Edoxaban API Price utilized in the formulation of products. Edoxaban API Price is not always fixed or binding as the Edoxaban Price is obtained through a variety of data sources. The Edoxaban Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Edoxaban manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Edoxaban, including repackagers and relabelers. The FDA regulates Edoxaban manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Edoxaban API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Edoxaban manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Edoxaban supplier is an individual or a company that provides Edoxaban active pharmaceutical ingredient (API) or Edoxaban finished formulations upon request. The Edoxaban suppliers may include Edoxaban API manufacturers, exporters, distributors and traders.
click here to find a list of Edoxaban suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Edoxaban DMF (Drug Master File) is a document detailing the whole manufacturing process of Edoxaban active pharmaceutical ingredient (API) in detail. Different forms of Edoxaban DMFs exist exist since differing nations have different regulations, such as Edoxaban USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Edoxaban DMF submitted to regulatory agencies in the US is known as a USDMF. Edoxaban USDMF includes data on Edoxaban's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Edoxaban USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Edoxaban suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Edoxaban Drug Master File in Korea (Edoxaban KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Edoxaban. The MFDS reviews the Edoxaban KDMF as part of the drug registration process and uses the information provided in the Edoxaban KDMF to evaluate the safety and efficacy of the drug.
After submitting a Edoxaban KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Edoxaban API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Edoxaban suppliers with KDMF on PharmaCompass.
A Edoxaban written confirmation (Edoxaban WC) is an official document issued by a regulatory agency to a Edoxaban manufacturer, verifying that the manufacturing facility of a Edoxaban active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Edoxaban APIs or Edoxaban finished pharmaceutical products to another nation, regulatory agencies frequently require a Edoxaban WC (written confirmation) as part of the regulatory process.
click here to find a list of Edoxaban suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Edoxaban as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Edoxaban API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Edoxaban as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Edoxaban and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Edoxaban NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Edoxaban suppliers with NDC on PharmaCompass.
Edoxaban Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Edoxaban GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Edoxaban GMP manufacturer or Edoxaban GMP API supplier for your needs.
A Edoxaban CoA (Certificate of Analysis) is a formal document that attests to Edoxaban's compliance with Edoxaban specifications and serves as a tool for batch-level quality control.
Edoxaban CoA mostly includes findings from lab analyses of a specific batch. For each Edoxaban CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Edoxaban may be tested according to a variety of international standards, such as European Pharmacopoeia (Edoxaban EP), Edoxaban JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Edoxaban USP).
