Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
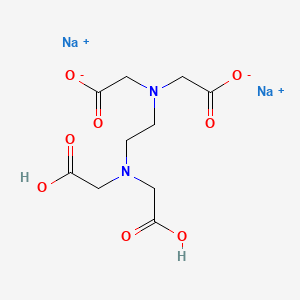

1. Acid, Edetic
2. Acid, Ethylenediaminetetraacetic
3. Acid, Ethylenedinitrilotetraacetic
4. Calcitetracemate, Disodium
5. Calcium Disodium Edetate
6. Calcium Disodium Versenate
7. Calcium Tetacine
8. Chelaton 3
9. Chromium Edta
10. Copper Edta
11. Coprin
12. Dicobalt Edta
13. Dinitrilotetraacetate, Disodium Ethylene
14. Dinitrilotetraacetate, Ethylene
15. Disodium Calcitetracemate
16. Disodium Edta
17. Disodium Ethylene Dinitrilotetraacetate
18. Disodium Versenate, Calcium
19. Distannous Edta
20. Edathamil
21. Edetate Disodium Calcium
22. Edetate, Calcium Disodium
23. Edetates
24. Edetic Acid
25. Edetic Acid, Calcium Salt
26. Edetic Acid, Calcium, Sodium Salt
27. Edetic Acid, Chromium Salt
28. Edetic Acid, Dipotassium Salt
29. Edetic Acid, Disodium Salt
30. Edetic Acid, Disodium Salt, Dihydrate
31. Edetic Acid, Disodium, Magnesium Salt
32. Edetic Acid, Disodium, Monopotassium Salt
33. Edetic Acid, Magnesium Salt
34. Edetic Acid, Monopotassium Salt
35. Edetic Acid, Monosodium Salt
36. Edetic Acid, Potassium Salt
37. Edetic Acid, Sodium Salt
38. Edta
39. Edta, Chromium
40. Edta, Copper
41. Edta, Dicobalt
42. Edta, Disodium
43. Edta, Distannous
44. Edta, Gallium
45. Edta, Magnesium Disodium
46. Edta, Potassium
47. Edta, Stannous
48. Ethylene Dinitrilotetraacetate
49. Ethylene Dinitrilotetraacetate, Disodium
50. Ethylenediaminetetraacetic Acid
51. Ethylenedinitrilotetraacetic Acid
52. Gallium Edta
53. Magnesium Disodium Edta
54. N,n'-1,2-ethanediylbis(n-(carboxymethyl)glycine)
55. Potassium Edta
56. Stannous Edta
57. Tetacine, Calcium
58. Tetracemate
59. Versenate
60. Versenate, Calcium Disodium
61. Versene
1. 139-33-3
2. Ethylenediaminetetraacetic Acid Disodium Salt
3. Disodium Edta
4. Edta Disodium
5. Edta Disodium Dihydrate
6. Disodium Ethylenediaminetetraacetate
7. Edta 2na
8. Disodium Ethylenediaminetetraacetic Acid
9. Disodium Dihydrogen Ethylenediaminetetraacetate
10. 6381-92-6
11. Ethylenediaminetetraacetic Acid Disodium Salt Solution
12. Disodium;2-[2-[bis(carboxymethyl)amino]ethyl-(carboxylatomethyl)amino]acetate
13. Ethylenediamine Tetraacetic Acid Disodium Salt
14. Edtadisodiumsalt
15. Eta Solution
16. Edta 2na Solution
17. Schembl33501
18. Akos015900960
19. Akos016016390
20. Cs-w019532
21. D3789
22. E0091
23. E0103
24. Ethylenediamine Tetra-acetic Acid Disodium Salt
25. N-[2-[bis(sodiooxycarbonylmethyl)amino]ethyl]iminobis(acetic Acid)
26. Sodium 2,2'-(2-(bis(carboxymethyl)amino)ethylazanediyl)diacetate
| Molecular Weight | 336.21 g/mol |
|---|---|
| Molecular Formula | C10H14N2Na2O8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 9 |
| Exact Mass | 336.05455397 g/mol |
| Monoisotopic Mass | 336.05455397 g/mol |
| Topological Polar Surface Area | 161 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 340 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Anticoagulants; Chelating Agents; Food Additives
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Endrate (Edetate Disodium Injection, USP) is indicated in selected patients for the emergency treatment of hypercalcemia and for the control of ventricular arrhythmias associated with digitalis toxicity. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Endrate (edetate disodium, anhydrous) injection, solution (May 2006). Available from, as of February 16, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=290c3e9c-c0c6-440a-1a9c-46e3e2b07a77
Disodium edentate is also used therapeutically as an anticoagulant as it will chelate calcium and prevent the coagulation of blood in vitro. Concentrations of 0.1% w/v are used in small volumes for hematological testing and 0.3% w/v in transfusions.
Rowe, R.C., Sheskey, P.J., Quinn, M.E.; (Eds.), Handbook of Pharmaceutical Excipients 6th edition Pharmaceutical Press, London, England 2009, p. 243
Disodium EDTA is used occasionally to terminate the effects of injected calcium, to antagonize digitalis toxicity, or to suppress tachyarrhythmias. /Former/
Cosmetic Ingredient Expert Review Panel; Final Final Report on the Safety Assessment of EDTA, Calcium, Disodium EDTA, Diammonium EDTA, Dipotassium EDTA, Disodium EDTA, TEA-EDTA, Tetrasodium EDTA, Tripotassium EDTA, Trisodium EDTA, HEDTA, and Trisodium HEDTA. International Journal of Toxicology 21 (S2): 95-142 (2002)
For more Therapeutic Uses (Complete) data for Disodium EDTA (8 total), please visit the HSDB record page.
/BOXED WARNING/ The use of this drug in any particular patient is recommended only when the severity of the clinical condition justifies the aggressive measures associated with this type of therapy.
US Natl Inst Health; DailyMed. Current Medication Information for Endrate (edetate disodium, anhydrous) injection, solution (May 2006). Available from, as of February 16, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=290c3e9c-c0c6-440a-1a9c-46e3e2b07a77
Clinical studies of edetate disodium did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
US Natl Inst Health; DailyMed. Current Medication Information for Endrate (edetate disodium, anhydrous) injection, solution (May 2006). Available from, as of February 16, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=290c3e9c-c0c6-440a-1a9c-46e3e2b07a77
Fatal medication errors have occurred that involve confusion between edetate calcium disodium (calcium EDTA) and edetate disodium (no longer commercially available in the US). Children and adults have mistakenly received edetate disodium instead of edetate calcium disodium; at least 5 deaths have occurred as a result of inadvertent administration of edetate disodium. Although both edetate calcium disodium and edetate disodium are heavy metal antagonists, the 2 drugs were originally approved by the US Food and Drug Administration (FDA) for different uses and have different effects; edetate disodium was formerly FDA approved for use in selected patients for the emergency treatment of hypercalcemia or for the control of ventricular arrhythmias associated with cardiac glycoside toxicity. Use of edetate disodium may result in a substantial, and sometimes fatal, decrease in serum calcium concentrations. In June 2008, FDA withdrew its prior approval for edetate disodium because of safety concerns following a review of the risk-benefit profile of the drug. FDA stated that it was not considering additional action regarding edetate calcium disodium at that time; most of the fatalities following administration of an EDTA drug have involved medication errors in which edetate disodium was administered instead of edetate calcium disodium. FDA has not received reports of any fatalities resulting from the administration of edetate calcium disodium that involve a medication error.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011
Edetate Disodium Injection is contraindicated in anuric patients. It is not indicated for the treatment of generalized arteriosclerosis associated with advancing age.
US Natl Inst Health; DailyMed. Current Medication Information for Endrate (edetate disodium, anhydrous) injection, solution (May 2006). Available from, as of February 16, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=290c3e9c-c0c6-440a-1a9c-46e3e2b07a77
For more Drug Warnings (Complete) data for Disodium EDTA (22 total), please visit the HSDB record page.
Anticoagulants
Agents that prevent BLOOD CLOTTING. (See all compounds classified as Anticoagulants.)
Calcium Chelating Agents
Substances that bind to and sequester CALCIUM ions. (See all compounds classified as Calcium Chelating Agents.)
Food Additives
Substances used in the processing or storage of foods or animal feed including ANTIOXIDANTS; FOOD PRESERVATIVES; FOOD COLORING AGENTS; FLAVORING AGENTS; ANTI-INFECTIVE AGENTS; EXCIPIENTS and other similarly used substances. Many of the same substances are used as PHARMACEUTIC AIDS. (See all compounds classified as Food Additives.)
After intravenous administration, the chelate formed is excreted in the urine with 50% appearing in 1 hour and over 95% in 24 hours.
US Natl Inst Health; DailyMed. Current Medication Information for Endrate (edetate disodium, anhydrous) injection, solution (May 2006). Available from, as of February 16, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=290c3e9c-c0c6-440a-1a9c-46e3e2b07a77
Disodium edentate ... /is/ poorly absorbed from the gastrointestinal tract and /is/ associated with few adverse effects when used as an excipient in pharmaceutical preparations.
Rowe, R.C., Sheskey, P.J., Quinn, M.E.; (Eds.), Handbook of Pharmaceutical Excipients 6th edition Pharmaceutical Press, London, England 2009, p. 243
Twenty male Sprague-Dawley rats were divided into four groups of five animals each. Rats in group 1 received ip injections of (14)C Disodium EDTA, group 2 received this compound on depilated skin, rats in group 3 received this compound on depilated and abraded skin (abraded every 2 or 3 cm over treated area), and group 4 was the control group. The specific activity of the (14)C Disodium EDTA was 21.6 mCi/mM and it was dissolved in saline to yield a final solution of 50 pCi/mL. Animals that received ip injections got 0.5 mL of this solution, or 25 pCi of (14)C Disodium EDTA. Animals that had the compound applied to the skin received 25 pCi of (14)C Disodium EDTA in the form of an ointment (modulan, mineral oil, petrolatum, cetyl alcohol 35:21 :25:12) spread over an area of 50 sq cm spread over a sheet of thin polyethylene. This sheet was taped to the trunk of each animal. A collar was fixed around the neck of the rats. All animals were decapitated 24 hours after treatment. The tissue distribution (per 100 mg wet organ weight) of (14)C Disodium EDTA 24 hours after ip administration was as follows: liver 577+/- 13, small intestine 631 +/- 25, large intestine 696 +/- 19, and kidney 1964 +/- 220. Twenty-four hours after application on normal skin the tissue distribution was as follows: liver 6 +/- 4, small intestine 99 +/- 22, large intestine 107 +/- 24, and kidneys 29 +/- 12. Twenty-four hours after application on abraded skin the tissue distribution was as follows: liver 139 +/- 34, small intestine 214 +/- 76, large intestine 309 +/- 115, and kidneys 222 +/- 30.
Cosmetic Ingredient Expert Review Panel; Final Final Report on the Safety Assessment of EDTA, Calcium, Disodium EDTA, Diammonium EDTA, Dipotassium EDTA, Disodium EDTA, TEA-EDTA, Tetrasodium EDTA, Tripotassium EDTA, Trisodium EDTA, HEDTA, and Trisodium HEDTA. International Journal of Toxicology 21 (S2): 95-142 (2002)
/Investigators/ reported that rats fed 0.5%, 1.0%, and 5.0% Disodium EDTA for 12 weeks excreted 82.2%, 44.5%, and 45.4%, respectively, of the ingested dose in the urine and feces. The feces contained 99.4%, 98.2%, and 97.5% of the excreted material and the urine contained 0.6%, 1.8%, and 2.5% of the material for the respective doses.
Cosmetic Ingredient Expert Review Panel; Final Final Report on the Safety Assessment of EDTA, Calcium, Disodium EDTA, Diammonium EDTA, Dipotassium EDTA, Disodium EDTA, TEA-EDTA, Tetrasodium EDTA, Tripotassium EDTA, Trisodium EDTA, HEDTA, and Trisodium HEDTA. International Journal of Toxicology 21 (S2): 95-142 (2002)
For more Absorption, Distribution and Excretion (Complete) data for Disodium EDTA (7 total), please visit the HSDB record page.
After intravenous administration, the chelate formed is excreted in the urine with 50% appearing in 1 hour and over 95% in 24 hours.
US Natl Inst Health; DailyMed. Current Medication Information for Endrate (edetate disodium, anhydrous) injection, solution (May 2006). Available from, as of February 16, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=290c3e9c-c0c6-440a-1a9c-46e3e2b07a77
Edetate disodium injection forms chelates with the cations of calcium and many divalent and trivalent metals. Because of its affinity for calcium, edetate disodium will produce a lowering of the serum calcium level during intravenous infusion. Slow infusion over a protracted period may cause mobilization of extracirculatory calcium stores. Edetate disodium exerts a negative inotropic effect upon the heart.
US Natl Inst Health; DailyMed. Current Medication Information for Endrate (edetate disodium, anhydrous) injection, solution (May 2006).
Edetate disodium likewise forms chelates with other polyvalent metals and produces increases in urinary excretion of magnesium, zinc and other trace elements. It does not form a chelate with potassium but may reduce the serum level and increase urinary loss of potassium.
US Natl Inst Health; DailyMed. Current Medication Information for Endrate (edetate disodium, anhydrous) injection, solution (May 2006). Available from, as of February 16, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=290c3e9c-c0c6-440a-1a9c-46e3e2b07a77
ABOUT THIS PAGE
55
PharmaCompass offers a list of Eta Solution API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Eta Solution manufacturer or Eta Solution supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Eta Solution manufacturer or Eta Solution supplier.
PharmaCompass also assists you with knowing the Eta Solution API Price utilized in the formulation of products. Eta Solution API Price is not always fixed or binding as the Eta Solution Price is obtained through a variety of data sources. The Eta Solution Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A EDTA-2NA.2H2O manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of EDTA-2NA.2H2O, including repackagers and relabelers. The FDA regulates EDTA-2NA.2H2O manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. EDTA-2NA.2H2O API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A EDTA-2NA.2H2O supplier is an individual or a company that provides EDTA-2NA.2H2O active pharmaceutical ingredient (API) or EDTA-2NA.2H2O finished formulations upon request. The EDTA-2NA.2H2O suppliers may include EDTA-2NA.2H2O API manufacturers, exporters, distributors and traders.
EDTA-2NA.2H2O Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of EDTA-2NA.2H2O GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right EDTA-2NA.2H2O GMP manufacturer or EDTA-2NA.2H2O GMP API supplier for your needs.
A EDTA-2NA.2H2O CoA (Certificate of Analysis) is a formal document that attests to EDTA-2NA.2H2O's compliance with EDTA-2NA.2H2O specifications and serves as a tool for batch-level quality control.
EDTA-2NA.2H2O CoA mostly includes findings from lab analyses of a specific batch. For each EDTA-2NA.2H2O CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
EDTA-2NA.2H2O may be tested according to a variety of international standards, such as European Pharmacopoeia (EDTA-2NA.2H2O EP), EDTA-2NA.2H2O JP (Japanese Pharmacopeia) and the US Pharmacopoeia (EDTA-2NA.2H2O USP).
