

Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
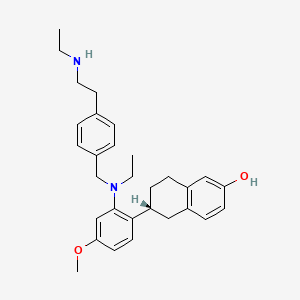

1. (6r)-6-(2-(ethyl((4-(2-(ethylamino)ethyl)phenyl)methyl)amino)-4-methoxyphenyl)-5,6,7,8-tetrahydronaphthalen-2-ol
1. 722533-56-4
2. Rad1901
3. Elacestrant [inn]
4. Elacestrant [usan]
5. Rad-1901
6. Er-306323
7. Fm6a2627a8
8. (6r)-6-[2-[ethyl-[[4-[2-(ethylamino)ethyl]phenyl]methyl]amino]-4-methoxyphenyl]-5,6,7,8-tetrahydronaphthalen-2-ol
9. (6r)-6-{2-[ethyl({4-[2-(ethylamino)ethyl]phenyl}methyl)amino]-4-methoxyphenyl}-5,6,7,8-tetrahydronaphthalen-2-ol
10. (2r)-2-(2-(ethyl-((4-(2-(ethylamino)ethyl)phenyl)methyl)amino)-4-methoxy-phenyl)tetralin-6-ol
11. (6r)-6-(2-(ethyl((4-(2- (ethylamino)ethyl)phenyl)methyl)amino)-4-methoxyphenyl)- 5,6,7,8-tetrahydronaphthalen-2-ol
12. 2-naphthalenol, 6-(2-(ethyl((4-(2-(ethylamino)ethyl)phenyl)methyl)amino)-4-methoxyphenyl)-5,6,7,8-tetrahydro-, (6r)-
13. Elacestrant (usan/inn)
14. Elacestrant [usan:inn]
15. Elacestrant [who-dd]
16. Schembl229431
17. Unii-fm6a2627a8
18. Chembl4297509
19. Bdbm349630
20. Dtxsid901045846
21. Glxc-26208
22. Ex-a5070
23. Us10208011, Compound Rad-
24. Mfcd30532693
25. Who 10247
26. Cs-6306
27. Db06374
28. Hy-19822
29. D11671
30. Q27278069
31. (r)-6-(2-(ethyl(4-(2-(ethylamino)ethyl)benzyl)amino)-4-methoxyphenyl)-5,6,7,8-tetrahydronaphthalen-2-ol
32. I0v
| Molecular Weight | 458.6 g/mol |
|---|---|
| Molecular Formula | C30H38N2O2 |
| XLogP3 | 6.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 10 |
| Exact Mass | 458.293328459 g/mol |
| Monoisotopic Mass | 458.293328459 g/mol |
| Topological Polar Surface Area | 44.7 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 578 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in menopause and female hormonal deficiencies/abnormalities.
RAD1901 is a novel selective estrogen receptor modulator(SERM). SERMs are small molecules that bind to and selectively modulate estrogen receptors. These molecules have the ability to stimulate or block estrogen's activity in different types of tissue, functioning as estrogen receptor agonists in some tissues and as estrogen receptor antagonists in others. RAD1901 has potential to reduce vasomotor symptoms, along with a simultaneous bone-protective effect, without stimulating breast or uterine tissues. RAD1901 is distinctive from other SERMs in its unique biological profile, combined with its significant ability to penetrate the blood-brain barrier, which enables RAD1901 to function as an estrogen agonist within the central nervous system and thereby relieve hot flashes.
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
About the Company : Granules is a vertically integrated fast-growing Pharmaceutical company headquartered in Hyderabad with best-in-class facilities & a commitment to operational excellence, quality, ...
 Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients & provides custom-made solutions.
Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients & provides custom-made solutions.
About the Company : Since its inception in 2003, Seqens has grown to become a global leader in pharmaceutical solutions and specialty ingredients. Seqens supports its customers in developing, scaling ...
About the Company : Since its founding in 1962, MOEHS has produced Active Pharmaceutical Ingredients (APIs) for the international pharmaceutical industry. Thanks to a business history of more than 50 ...
About the Company : Established in May 2012, Shandong Loncom Pharmaceutical operates as a fully owned subsidiary of Shandong Bestcomm Pharmaceutical Co., Ltd. Situated in the Qihe Economic Development...
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
