

Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
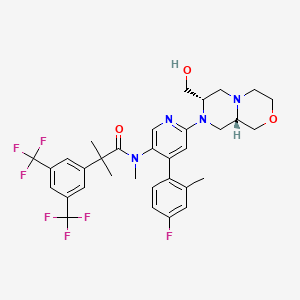

1. Unii-nzw2bow35n
2. 929046-33-3
3. Nt-814
4. Elinzanetant [inn]
5. Elinzanetant [usan]
6. Nzw2bow35n
7. Bay3427080
8. Bay-3427080
9. 2-(3,5-bis(trifluoromethyl)phenyl)-n-(4-(4-fluoro-2-methylphenyl)-6-((7s,9as)-7-(hydroxymethyl)hexahydropyrazino(2,1-c)(1,4)oxazin-8(1h)-yl)-3-pyridinyl)-n,2-dimethylpropanamide
10. 2-[3,5-bis(trifluoromethyl)phenyl]-n-{4-(4-fluoro-2-methylphenyl)-6-[(7s,9as)-7-(hydroxymethyl)hexahydropyrazino[2,1-c][1,4]oxazin-8(1h)-yl]-3-pyridinyl}-n,2-dimethylpropanamide
11. Elinzanetant [who-dd]
12. Schembl303180
13. Chembl4802157
14. Dtxsid101337049
15. Ex-a6225
16. Who 10952
17. Hy-109171
18. Cs-0116361
19. 2-[3,5-bis(trifluoromethyl)phenyl]-n-{4-(4-fluoro-2-methylphenyl)-6-[(7s,9as)-7-(hydroxymethyl)hexahydropyrazino[2,1-c][1,4]oxazin-8(1h)-yl]pyridin-3-yl}-n,2-dimethylpropanamide
20. Benzamide, 3-(5-methyl-2-tbenzeneacetamide, N-(4-(4-fluoro-2-methylphenyl)-6-((7s,9as)-hexahydro-7-(hydroxymethyl)pyrazino(2,1-c)(1,4)oxazin-8(1h)-yl)-3-pyridinyl)-n,.alpha.,.alpha.-trimethyl-3,5-bis(trifluoromethyl)-
21. Benzamide, 3-(5-methyl-2-tbenzeneacetamide, N-(4-(4-fluoro-2-methylphenyl)-6-((7s,9as)-hexahydro-7-(hydroxymethyl)pyrazino(2,1-c)(1,4)oxazin-8(1h)-yl)-3-pyridinyl)-n,alpha,alpha-trimethyl-3,5-bis(trifluoromethyl)-
22. N-[6-[(7s,9as)-7-(hydroxymethyl)-3,4,6,7,9,9a-hexahydro-1h-pyrazino[2,1-c][1,4]oxazin-8-yl]-4-(4-fluoro-2-methylphenyl)pyridin-3-yl]-2-[3,5-bis(trifluoromethyl)phenyl]-n,2-dimethylpropanamide
| Molecular Weight | 668.6 g/mol |
|---|---|
| Molecular Formula | C33H35F7N4O3 |
| XLogP3 | 5.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 6 |
| Exact Mass | 668.25973813 g/mol |
| Monoisotopic Mass | 668.25973813 g/mol |
| Topological Polar Surface Area | 69.1 Ų |
| Heavy Atom Count | 47 |
| Formal Charge | 0 |
| Complexity | 1060 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
BAY3427080 (elinzanetant) is the first dual neurokinin-1,3 (NK-1,3) receptor antagonist, which is being evaluated for the treatment of moderate to severe vasomotor symptoms.
Lead Product(s): Elinzanetant
Therapeutic Area: Obstetrics/Gynecology (Women’s Health) Brand Name: BAY3427080
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable September 10, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Elinzanetant
Therapeutic Area : Obstetrics/Gynecology (Women’s Health)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
OASIS 3 Study Supports Efficacy and Safety of Elinzanetant for Menopause Symptoms
Details : BAY3427080 (elinzanetant) is the first dual neurokinin-1,3 (NK-1,3) receptor antagonist, which is being evaluated for the treatment of moderate to severe vasomotor symptoms.
Brand Name : BAY3427080
Molecule Type : Small molecule
Upfront Cash : Not Applicable
September 10, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
BAY3427080 (elinzanetant) is the first dual neurokinin-1,3 (NK-1,3) receptor antagonist, which is being evaluated for the treatment of moderate to severe vasomotor symptoms.
Lead Product(s): Elinzanetant
Therapeutic Area: Obstetrics/Gynecology (Women’s Health) Brand Name: BAY3427080
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable September 04, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Elinzanetant
Therapeutic Area : Obstetrics/Gynecology (Women’s Health)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Bayer to Present Data from Phase III OASIS 3 Study on Elinzanetant for Menopausal Symptoms
Details : BAY3427080 (elinzanetant) is the first dual neurokinin-1,3 (NK-1,3) receptor antagonist, which is being evaluated for the treatment of moderate to severe vasomotor symptoms.
Brand Name : BAY3427080
Molecule Type : Small molecule
Upfront Cash : Not Applicable
September 04, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
BAY3427080 (elinzanetant) is the first dual neurokinin-1,3 (NK-1,3) receptor antagonist, which is being evaluated for the treatment of moderate to severe vasomotor symptoms.
Lead Product(s): Elinzanetant
Therapeutic Area: Obstetrics/Gynecology (Women’s Health) Brand Name: BAY3427080
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 01, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Elinzanetant
Therapeutic Area : Obstetrics/Gynecology (Women’s Health)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Bayer Submits NDA for Elinzanetant for Menopause Symptoms to U.S. FDA
Details : BAY3427080 (elinzanetant) is the first dual neurokinin-1,3 (NK-1,3) receptor antagonist, which is being evaluated for the treatment of moderate to severe vasomotor symptoms.
Brand Name : BAY3427080
Molecule Type : Small molecule
Upfront Cash : Not Applicable
August 01, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
BAY3427080 (elinzanetant) is the first dual neurokinin-1,3 (NK-1,3) receptor antagonist, which is being evaluated for the treatment of moderate to severe vasomotor symptoms.
Lead Product(s): Elinzanetant
Therapeutic Area: Obstetrics/Gynecology (Women’s Health) Brand Name: BAY3427080
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable May 16, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Elinzanetant
Therapeutic Area : Obstetrics/Gynecology (Women’s Health)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Elinzanetant Reduces Hot Flashes In Menopausal Women
Details : BAY3427080 (elinzanetant) is the first dual neurokinin-1,3 (NK-1,3) receptor antagonist, which is being evaluated for the treatment of moderate to severe vasomotor symptoms.
Brand Name : BAY3427080
Molecule Type : Small molecule
Upfront Cash : Not Applicable
May 16, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
BAY3427080 (elinzanetant) is the first dual neurokinin-1,3 (NK-1,3) receptor antagonist, which is being evaluated for the treatment of moderate to severe vasomotor symptoms (VMS, also known as hot flashes.
Lead Product(s): Elinzanetant
Therapeutic Area: Obstetrics/Gynecology (Women’s Health) Brand Name: BAY3427080
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable May 08, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Elinzanetant
Therapeutic Area : Obstetrics/Gynecology (Women’s Health)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : BAY3427080 (elinzanetant) is the first dual neurokinin-1,3 (NK-1,3) receptor antagonist, which is being evaluated for the treatment of moderate to severe vasomotor symptoms (VMS, also known as hot flashes.
Brand Name : BAY3427080
Molecule Type : Small molecule
Upfront Cash : Not Applicable
May 08, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
BAY3427080 (elinzanetant) is the first dual neurokinin-1,3 (NK-1,3) receptor antagonist, which is being evaluated for the treatment of moderate to severe vasomotor symptoms (VMS, also known as hot flashes.
Lead Product(s): Elinzanetant
Therapeutic Area: Obstetrics/Gynecology (Women’s Health) Brand Name: BAY3427080
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable March 19, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Elinzanetant
Therapeutic Area : Obstetrics/Gynecology (Women’s Health)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : BAY3427080 (elinzanetant) is the first dual neurokinin-1,3 (NK-1,3) receptor antagonist, which is being evaluated for the treatment of moderate to severe vasomotor symptoms (VMS, also known as hot flashes.
Brand Name : BAY3427080
Molecule Type : Small molecule
Upfront Cash : Not Applicable
March 19, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
BAY-3427080 (elinzanetant) is a first dual neurokinin-1,3 (NK-1,3) receptor antagonist, which is in phase 2 clinical development for the treatment of sleep disturbances associated with menopause.
Lead Product(s): Elinzanetant
Therapeutic Area: Obstetrics/Gynecology (Women’s Health) Brand Name: BAY-3427080
Study Phase: Phase IIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable January 08, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Elinzanetant
Therapeutic Area : Obstetrics/Gynecology (Women’s Health)
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : BAY-3427080 (elinzanetant) is a first dual neurokinin-1,3 (NK-1,3) receptor antagonist, which is in phase 2 clinical development for the treatment of sleep disturbances associated with menopause.
Brand Name : BAY-3427080
Molecule Type : Small molecule
Upfront Cash : Not Applicable
January 08, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
BAY-3427080 (elinzanetant) is a first dual neurokinin-1,3 (NK-1,3) receptor antagonist, in late-stage clinical development for the non-hormonal treatment of moderate to severe VMS associated with menopause, administered orally once daily.
Lead Product(s): Elinzanetant
Therapeutic Area: Obstetrics/Gynecology (Women’s Health) Brand Name: BAY-3427080
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable January 08, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Elinzanetant
Therapeutic Area : Obstetrics/Gynecology (Women’s Health)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Bayer’s Elinzanetant Meets All Primary and Key Secondary Endpoints in Pivotal OASIS 1 and 2 Phas...
Details : BAY-3427080 (elinzanetant) is a first dual neurokinin-1,3 (NK-1,3) receptor antagonist, in late-stage clinical development for the non-hormonal treatment of moderate to severe VMS associated with menopause, administered orally once daily.
Brand Name : BAY-3427080
Molecule Type : Small molecule
Upfront Cash : Not Applicable
January 08, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
BAY3427080 (elinzanetant) is an oral, once-daily, non-hormonal, selective neurokinin-1,3 (NK-1,3) receptor antagonist that is being studied for the reduction of vasomotor symptoms (VMS).
Lead Product(s): Elinzanetant
Therapeutic Area: Obstetrics/Gynecology (Women’s Health) Brand Name: BAY3427080
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable February 02, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Elinzanetant
Therapeutic Area : Obstetrics/Gynecology (Women’s Health)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : BAY3427080 (elinzanetant) is an oral, once-daily, non-hormonal, selective neurokinin-1,3 (NK-1,3) receptor antagonist that is being studied for the reduction of vasomotor symptoms (VMS).
Brand Name : BAY3427080
Molecule Type : Small molecule
Upfront Cash : Not Applicable
February 02, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
BAY3427080 (elinzanetant) is a non-hormonal, orally administered, dual neurokinin-1,3 receptor antagonist currently in clinical development for the treatment of vasomotor symptoms during menopause.
Lead Product(s): Elinzanetant
Therapeutic Area: Obstetrics/Gynecology (Women’s Health) Brand Name: BAY3427080
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable November 07, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Elinzanetant
Therapeutic Area : Obstetrics/Gynecology (Women’s Health)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : BAY3427080 (elinzanetant) is a non-hormonal, orally administered, dual neurokinin-1,3 receptor antagonist currently in clinical development for the treatment of vasomotor symptoms during menopause.
Brand Name : BAY3427080
Molecule Type : Small molecule
Upfront Cash : Not Applicable
November 07, 2022

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
