
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
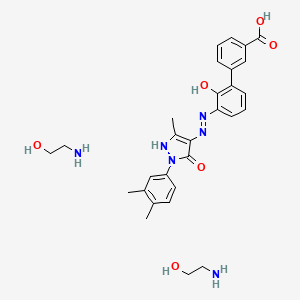

1. (1,1'-biphenyl)-3-carboxylic Acid, 3'-((2z)-(1-(3,4-dimethylphenyl)-1,5-dihydro-3-methyl-5-oxo-4h-pyrazol-4-ylidene)hydrazino)-2'-hydroxy-
2. Ddl-701
3. Eltrombopag
4. Promacta
5. Revolade
6. Sb-497 115
7. Sb-497-115
8. Sb497115
1. 496775-62-3
2. Revolade
3. Promacta
4. Eltrombopag (olamine)
5. Unii-4u07f515lg
6. Sb-497115-gr
7. Eltrombopag Diethanolamine Salt
8. 496775-62-3 (olamine)
9. Eltrombopag (as Olamine)
10. 4u07f515lg
11. Sb-497115gr
12. Eltrombopag Compd With 2-aminoethanol (1:2)
13. Eltrombopag Diethanolamine Salt;sb-497115gr
14. (z)-3'-(2-(1-(3,4-dimethylphenyl)-3-methyl-5-oxo-1,5-dihydro-4h-pyrazol-4-ylidene)hydrazinyl)-2'-hydroxy-[1,1'-biphenyl]-3-carboxyiic Acid;2-aminoethan-1-ol (1:2)
15. 3'-((2z)-2-(1-(3,4-dimethylphenyl)-3-methyl-5-oxo-1,5-dihydro-4h-pyrazol-4-ylidene)diazanyl)-2'-hydroxybiphenyl-3-carboxylic Acid Compound With 2-aminoethanol (1:2)
16. Promacta Olamine
17. Eltrombopagolamine
18. Eltrombopag Olamine [usan:jan]
19. Promacta (tn)
20. Sb 497115gr
21. Schembl210183
22. Amy267
23. Chembl3989691
24. Schembl16207741
25. Schembl23458316
26. Eltrombopag Olamine (jan/usan)
27. Eltrombopag Olamine [jan]
28. Eltrombopag Olamine [usan]
29. Bcp07055
30. Eltrombopag Olamine [mart.]
31. Eltrombopag Olamine [who-dd]
32. Hy-15306a
33. Mfcd22380664
34. S2229
35. Akos025396658
36. Akos037515856
37. Ccg-270074
38. Cs-1566
39. Sb19102
40. Eltrombopag Olamine [orange Book]
41. 2-aminoethan-1-ol Hemi((e)-3'-(2-(2-(3,4-dimethylphenyl)-5-methyl-3-oxo-2,3-dihydro-1h-pyrazol-4-yl)hydrazono)-2'-oxo-2',3'-dihydro-[1,1'-biphenyl]-3-carboxylate)
42. Ac-26286
43. Bs-17370
44. Ft-0773802
45. D03978
46. A904098
47. Q27260489
48. Eltrombopag Compd With 2-aminoethanol (1:2) [mi]
49. (1,1'-biphenyl)-3-carboxylic Acid, 3'-((2z)-(1-(3,4-dimethylphenyl)-1,5-dihydro-3-methyl-5-oxo-4h-pyrazol-4-ylidene)hydrazino)-2'-hydroxy, Compound With 2-aminoethanol (1:2)
50. (1,1'-biphenyl)-3-carboxylic Acid, 3'-((2z)-(1-(3,4-dimethylphenyl)-1,5-dihydro-3-methyl-5-oxo-4h-pyrazol-4-ylidene)hydrazino)-2'-hydroxy-, Compound With 2-aminoethanol (1:2)
51. (1,1'-biphenyl)-3-carboxylic Acid, 3'-(2-(1-(3,4-dimethylphenyl)-4,5-dihydro-3-methyl-5-oxo-1h-pyrazol-4-yl)diazenyl)-2'-hydroxy-, Compd. With 2-aminoethanol (1:2)
52. 2-aminoethan-1-ol Hemi((z)-3'-(2-(1-(3,4-dimethylphenyl)-3-methyl-5-oxo-1,5-dihydro-4h-pyrazol-4-ylidene)hydrazinyl)-2'-hydroxy-[1,1'-biphenyl]-3-carboxylate)
53. 2-aminoethanol (e)-3'-(2-(1-(3,4-dimethylphenyl)-3-methyl-5-oxo-1h-pyrazol-4(5h)-ylidene)hydrazinyl)-2'-hydroxy-[1,1'-biphenyl]-3-carboxylate
54. 2-aminoethanol;3-[3-[[2-(3,4-dimethylphenyl)-5-methyl-3-oxo-1h-pyrazol-4-yl]diazenyl]-2-hydroxyphenyl]benzoic Acid
55. 3'-[(2z)-[1-(3,4-dimethylphenyl)-1,5-dihydro-3-methyl-5-oxo-4h-pyrazol-4-ylidene]hydrazino]-2'-hydroxy-[1,1'-biphenyl]-3-carboxylic Acid Bis-(monoethanolamine)
56. 3'-[(2z)-[1-(3,4-dimethylphenyl)-1,5-dihydro-3-methyl-5-oxo-4h-pyrazol4-ylidene]hydrazino]-2'-hydroxy-[1,1'-biphenyl]-3-carboxylic Acid Bis-(monoethanolamine)
57. 3'-{(2z)-2-[1-(3,4-dimethylphenyl)-3-methyl-5-oxo-1,5-dihydro-4h-pyrazol-4-ylidene]hydrazino}-2'-hydroxy-3-biphenylcarboxylic Acid 2-aminoethanol (1:2)
| Molecular Weight | 564.6 g/mol |
|---|---|
| Molecular Formula | C29H36N6O6 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 7 |
| Exact Mass | 564.26963289 g/mol |
| Monoisotopic Mass | 564.26963289 g/mol |
| Topological Polar Surface Area | 207 Ų |
| Heavy Atom Count | 41 |
| Formal Charge | 0 |
| Complexity | 822 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Revolade is indicated for the treatment of adult patients with primary immune thrombocytopenia (ITP) who are refractory to other treatments (e. g. corticosteroids, immunoglobulins) (see sections 4. 2 and 5. 1).
Revolade is indicated for the treatment of paediatric patients aged 1 year and above with primary immune thrombocytopenia (ITP) lasting 6 months or longer from diagnosis and who are refractory to other treatments (e. g. corticosteroids, immunoglobulins) (see sections 4. 2 and 5. 1).
Revolade is indicated in adult patients with chronic hepatitis C virus (HCV) infection for the treatment of thrombocytopenia, where the degree of thrombocytopenia is the main factor preventing the initiation or limiting the ability to maintain optimal interferon-based therapy (see sections 4. 4 and 5. 1).
Revolade is indicated in adult patients with acquired severe aplastic anaemia (SAA) who were either refractory to prior immunosuppressive therapy or heavily pretreated and are unsuitable for haematopoietic stem cell transplantation (see section 5. 1).
B02BX05
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
GDUFA
DMF Review : Complete
Rev. Date : 2024-02-13
Pay. Date : 2024-01-08
DMF Number : 39129
Submission : 2023-12-31
Status : Active
Type : II
 ChemWerth works in generic API development & supply, non-infringement patent strategy development and regulatory support.
ChemWerth works in generic API development & supply, non-infringement patent strategy development and regulatory support.
GDUFA
DMF Review : Complete
Rev. Date : 2022-10-25
Pay. Date : 2022-09-28
DMF Number : 37269
Submission : 2022-08-29
Status : Active
Type : II
 Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
GDUFA
DMF Review : Complete
Rev. Date : 2023-11-08
Pay. Date : 2023-09-28
DMF Number : 38008
Submission : 2023-03-31
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2021-11-16
Pay. Date : 2021-09-28
DMF Number : 36333
Submission : 2021-09-29
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37134
Submission : 2022-05-31
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2014-04-28
Pay. Date : 2013-02-01
DMF Number : 26746
Submission : 2012-12-20
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2017-10-31
Pay. Date : 2017-10-10
DMF Number : 28842
Submission : 2014-12-20
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2017-06-01
Pay. Date : 2017-04-21
DMF Number : 31667
Submission : 2017-04-29
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2022-04-29
Pay. Date : 2022-03-01
DMF Number : 36769
Submission : 2022-03-24
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 35520
Submission : 2021-02-18
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Regulatory Info : EU Dossier Readiness-Q2 2019
Registration Country : Germany
Brand Name :
Dosage Form : Film Coated Tablet
Dosage Strength : 12.5MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : EU Dossier Readiness-Q2 2019
Registration Country : Germany
Regulatory Info : EU Dossier Readiness-Q2 2019
Registration Country : Germany
Brand Name :
Dosage Form : Film Coated Tablet
Dosage Strength : 25MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : EU Dossier Readiness-Q2 2019
Registration Country : Germany
Regulatory Info : EU Dossier Readiness-Q2 2019
Registration Country : Germany
Brand Name :
Dosage Form : Film Coated Tablet
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : EU Dossier Readiness-Q2 2019
Registration Country : Germany
Regulatory Info : EU Dossier Readiness-Q2 2019
Registration Country : Germany
Brand Name :
Dosage Form : Film Coated Tablet
Dosage Strength : 75MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : EU Dossier Readiness-Q2 2019
Registration Country : Germany
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Revolade
Dosage Form : Filmtable
Dosage Strength : 25mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Revolade
Dosage Form : Filmtable
Dosage Strength : 25mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Revolade
Dosage Form : Filmtable
Dosage Strength : 50mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Revolade
Dosage Form : Filmtable
Dosage Strength : 50mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Revolade
Dosage Form : Filmtabl
Dosage Strength : 75mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Revolade
Dosage Form : Filmtable
Dosage Strength : 12.5mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?