

Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
API
0
FDF
0
FDA Orange Book

0
Canada

0
Australia

DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
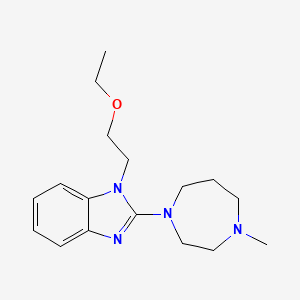

1. 1-(2-ethoxyethyl)-2-(4-methyl-1-homopiperazinyl)benzimidazole Difumarate
2. Emadine
3. Emedastine Difumarate
4. Kb 2413
5. Kb-2413
6. Kg 2413
7. Kg-2413
1. 87233-61-2
2. Emadine
3. Emedastine [inn]
4. Emedastina
5. Emedastinum
6. Emedastinum [inn-latin]
7. Emedastina [inn-spanish]
8. 1-(2-ethoxyethyl)-2-(4-methyl-1,4-diazepan-1-yl)-1h-benzo[d]imidazole
9. 1-(2-ethoxyethyl)-2-(4-methyl-1,4-diazepan-1-yl)benzimidazole
10. Emedastine (inn)
11. 1-(2-ethoxyethyl)-2-(hexahydro-4-methyl-1h-1,4-diazepin-1-yl)benzimidazole
12. Emadine (tn)
13. Chembl594
14. 1-[2-(ethoxy)ethyl]-2-(4-methyl-1-homopiperazinyl)benzimidazole
15. 9j1h7y9ojv
16. Chebi:4779
17. 1-(2-ethoxy-ethyl)-2-(4-methyl-[1,4]diazepan-1-yl)-1h-benzoimidazole
18. 1-(2-ethoxyethyl)-2-(4-methyl-1,4-diazepan-1-yl)-1h-1,3-benzodiazole
19. Emedastine [inn:ban]
20. Ncgc00181341-01
21. Unii-9j1h7y9ojv
22. 1-methyl-4-(1-(2-ethoxyethyl)-1h-benzimidazo)-2-yl)(1,4)diazepane
23. Emedastine [mi]
24. Emedastine [vandf]
25. Emedastine [who-dd]
26. Schembl29770
27. Emedastine [ema Epar]
28. Gtpl7174
29. Dtxsid7048243
30. Hms3886o14
31. Amy25237
32. Bcp20085
33. Ex-a1371
34. Zinc1530912
35. 1-(2-ethoxyethyl)-2-(4-methyl-1,4-diazepan-1-yl)-1h-benzimidazole
36. Bdbm50019624
37. Mfcd00865647
38. S5659
39. Akos037515523
40. Ccg-267493
41. Db01084
42. 1h-benzimidazole, 1-(2-ethoxyethyl)-2-(hexahydro-4-methyl-1h-1,4-diazepin-1-yl)-
43. Ac-35544
44. Bs-17691
45. Hy-108411
46. Cs-0028590
47. Ft-0693271
48. C07785
49. D07890
50. D81889
51. 233e612
52. L001093
53. Q5370305
54. Brd-k15010214-313-01-6
55. 1-(2-ethoxyethyl)-2-(4-methyl-1-homopiperazinyl) Benzimidazole
56. 1-methyl-4-(1-(2-ethoxyethyl)-1h-benzimidazol-2-yl)[1,4]diazepane
| Molecular Weight | 302.4 g/mol |
|---|---|
| Molecular Formula | C17H26N4O |
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Exact Mass | 302.21066147 g/mol |
| Monoisotopic Mass | 302.21066147 g/mol |
| Topological Polar Surface Area | 33.5 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 341 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Emadine |
| PubMed Health | Emedastine Difumarate (Into the eye) |
| Drug Classes | Ophthalmologic Agent |
| Drug Label | EMADINE (emedastine difumarate ophthalmic solution) 0.05% is a sterile ophthalmic solution containing emedastine, a relatively selective, H1-receptor antagonist for topical administration to the eyes. Emedastine difumarate is a white, crystalline,... |
| Active Ingredient | Emedastine difumarate |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Alcon |
| 2 of 2 | |
|---|---|
| Drug Name | Emadine |
| PubMed Health | Emedastine Difumarate (Into the eye) |
| Drug Classes | Ophthalmologic Agent |
| Drug Label | EMADINE (emedastine difumarate ophthalmic solution) 0.05% is a sterile ophthalmic solution containing emedastine, a relatively selective, H1-receptor antagonist for topical administration to the eyes. Emedastine difumarate is a white, crystalline,... |
| Active Ingredient | Emedastine difumarate |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Alcon |
For the temporary relief of the signs and symptoms of allergic conjunctivitis.
FDA Label
Symptomatic treatment of seasonal allergic conjunctivitis.
Emedastine is a relatively selective H1-receptor antagonist.
Anti-Allergic Agents
Agents that are used to treat allergic reactions. Most of these drugs act by preventing the release of inflammatory mediators or inhibiting the actions of released mediators on their target cells. (From AMA Drug Evaluations Annual, 1994, p475) (See all compounds classified as Anti-Allergic Agents.)
Histamine H1 Antagonists
Drugs that selectively bind to but do not activate histamine H1 receptors, thereby blocking the actions of endogenous histamine. Included here are the classical antihistaminics that antagonize or prevent the action of histamine mainly in immediate hypersensitivity. They act in the bronchi, capillaries, and some other smooth muscles, and are used to prevent or allay motion sickness, seasonal rhinitis, and allergic dermatitis and to induce somnolence. The effects of blocking central nervous system H1 receptors are not as well understood. (See all compounds classified as Histamine H1 Antagonists.)
S01GX06
S01GX06
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
S - Sensory organs
S01 - Ophthalmologicals
S01G - Decongestants and antiallergics
S01GX - Other antiallergics
S01GX06 - Emedastine
Absorption
Ophthalmic use of emedastine usually does not produce measurable plasma concentrations.
Route of Elimination
Following oral administration, approximately 44% of the total dose can be recovered in the urine over the 24-hour period, with only 3.6% of the dose excreted as unchanged form. Two primary metabolites, 5- and 6-hydroxyemedastine, are excreted in the urine as both free and conjugated forms.
Two primary metabolites, 5-hydroxyemedastine and 6-hydroxyemedastine, are excreted in the urine as both free and conjugated forms. Minor metabolites include the 5'-oxoanalogs of 5-hydroxyemedastine and 6-hydroxy-emedastine and the N-oxide.
The elimination half-life in the plasma is 3-4 hours following oral administration.
Emedastine is a relatively selective, histamine H1 antagonist. In vitro examinations of emedastine's affinity for histamine receptors demonstrate relative selectivity for the H1 histamine receptor. In vivo studies have shown concentration-dependent inhibition of histamine-stimulated vascular permeability in the conjunctiva following topical ocular administration. Emedastine appears exert negligible effects on adrenergic, dopaminergic and serotonin receptors.

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
About the Company : Refarmed Chemicals is a fully integrated Swiss-based marketing company with extensive experience in the generics industry. Our global market activity and strategic offices located ...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Turkey
Brand Name : EMEKSAT
Dosage Form : EYE DROPS
Dosage Strength : 0.5MG/ML
Packaging : 5 ML/BOTTLE
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Turkey

Regulatory Info :
Registration Country : Switzerland
Brand Name : Emadine
Dosage Form : Gtt Opht
Dosage Strength :
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : Emadine
Dosage Form : OPD
Dosage Strength : 0.5mg/ml
Packaging : 5X1mg/ml
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Excipients by Applications
Global Sales Information

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
