
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
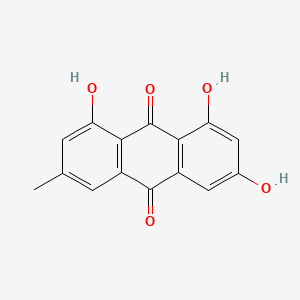

1. Archin
2. Casanthranol
3. Emodin, Frangula
4. Emodin, Rheum
5. Frangula Emodin
6. Frangulic Acid
7. Peristim
8. Rheum Emodin
1. 518-82-1
2. Emodol
3. Frangula Emodin
4. Schuttgelb
5. 1,3,8-trihydroxy-6-methylanthracene-9,10-dione
6. Rheum Emodin
7. Archin
8. Frangulic Acid
9. 3-methyl-1,6,8-trihydroxyanthraquinone
10. Persian Berry Lake
11. 1,3,8-trihydroxy-6-methylanthraquinone
12. 6-methyl-1,3,8-trihydroxyanthraquinone
13. 1,3,8-trihydroxy-6-methyl-9,10-anthraquinone
14. 1,3,8-trihydroxy-6-methyl-9,10-anthracenedione
15. C.i. Natural Yellow 14
16. 9,10-anthracenedione, 1,3,8-trihydroxy-6-methyl-
17. Alatinone
18. Rheum-emodin
19. 4,5,7-trihydroxy-2-methylanthraquinone
20. Frangulinic Acid
21. C.i. 75440
22. Nsc 408120
23. Nsc 622947
24. Anthraquinone, 1,3,8-trihydroxy-6-methyl-
25. Nsc408120
26. Ka46rni6hn
27. Chembl289277
28. Anthraquinone, 6-methyl-1,3,8-trihydroxy-
29. Chebi:42223
30. 1,3,8-trihydroxy-6-methylanthra-9,10-quinone
31. Nsc622947
32. Nsc-408120
33. Nsc-622947
34. Dsstox_cid_5231
35. Dsstox_rid_77709
36. 6-methyl-1,3,8-trihydroxy-9,10-anthracenedione
37. Dsstox_gsid_25231
38. Emo
39. 1,8-trihydroxy-6-methylanthraquinone
40. Cas-518-82-1
41. Smr000326798
42. Ccris 3528
43. Hsdb 7093
44. Sr-01000075615
45. Einecs 208-258-8
46. Unii-ka46rni6hn
47. Brn 1888141
48. Emdoin
49. Ai3-38286
50. 3bqc
51. Emodin,(s)
52. Mfcd00001207
53. Spectrum_001954
54. 1f0q
55. 3ed0
56. Specplus_000332
57. Emodin [hsdb]
58. Emodin [inci]
59. 1,3,8-tri-hydroxy-6-methyl-anthra-quinone
60. Emodin [usp-rs]
61. Spectrum2_000895
62. Spectrum3_000742
63. Spectrum4_001757
64. Spectrum5_000614
65. Emodin [mi]
66. Lopac-e-7881
67. Emodin, Analytical Standard
68. Ncimech_000049
69. Lopac0_000552
70. Bspbio_002324
71. Kbiogr_002234
72. Kbioss_002508
73. 1,3,8-trihydroxy-6-methyl-anthracene-9,10-dione
74. Mls000563068
75. Mls001066370
76. Mls004257392
77. Mls006011712
78. Divk1c_006428
79. Schembl177689
80. Spbio_000710
81. Megxp0_000460
82. Dtxsid5025231
83. Acon1_001939
84. Bdbm11318
85. Kbio1_001372
86. Kbio2_002500
87. Kbio2_005068
88. Kbio2_007636
89. Kbio3_001544
90. Emodin - Cas 518-82-1
91. Hms2230k22
92. Hms3261p05
93. Hms3373b16
94. Hms3655h22
95. 1,3,8-trihydroxy-6-methyl-9,10-dihydroanthracene-9,10-dione
96. Act03256
97. Bcp18372
98. Ex-a6778
99. Tnp00318
100. Zinc3824868
101. 9, 1,3,8-trihydroxy-6-methyl-
102. Tox21_202999
103. Tox21_303218
104. Tox21_500552
105. Ccg-35263
106. Lmpk13040008
107. S2295
108. Stl581876
109. 3-methyl-1,8-trihydroxyanthraquinone
110. 4,7-trihydroxy-2-methylanthraquinone
111. Akos003348641
112. Ac-1004
113. Cs-1412
114. Db07715
115. Ks-5189
116. Lp00552
117. Sdccgsbi-0050535.p004
118. Anthraquinone,3,8-trihydroxy-6-methyl-
119. Smp2_000211
120. Ncgc00015420-01
121. Ncgc00015420-02
122. Ncgc00015420-03
123. Ncgc00015420-04
124. Ncgc00015420-05
125. Ncgc00015420-06
126. Ncgc00015420-07
127. Ncgc00015420-08
128. Ncgc00015420-09
129. Ncgc00015420-22
130. Ncgc00091540-01
131. Ncgc00091540-02
132. Ncgc00091540-03
133. Ncgc00091540-04
134. Ncgc00091540-05
135. Ncgc00257090-01
136. Ncgc00260544-01
137. Ncgc00261237-01
138. 1,3,8-trihydroxy-6-methyl-anthraquinone
139. Hy-14393
140. Nci60_003906
141. E0500
142. Eu-0100552
143. Ft-0606539
144. Ft-0667846
145. N1854
146. Sw219906-1
147. 1,8-trihydroxy-6-methyl-9,10-anthraquinone
148. Emodin, From Frangula Bark, >=90% (hplc)
149. E 7881
150. K00056
151. Emodin; 6-methyl-1,3,8-trihydroxyanthraquinone
152. 1,3, 8-trihydroxy-6-methyl-9,10-anthraquinone
153. 1,3,8-trihydroxy-6-methylanthra-9,10-quinone #
154. 518e821
155. A828825
156. Q-100581
157. Q4348178
158. Sr-01000075615-1
159. Sr-01000075615-6
160. Brd-k58685305-001-03-0
161. 1,3,8-trihydroxy-6-methyl-9,10-anthracenedione, 9ci
162. 9,10-anthracenedione, 1,3,8-trihydroxy-6-methyl- (9ci)
163. Emodin, United States Pharmacopeia (usp) Reference Standard
164. 1,3,8-trihydroxy-6-methyl-anthracene-9,10-dione;3-methyl-1,6,8-trihydroxyanthraquinone
| Molecular Weight | 270.24 g/mol |
|---|---|
| Molecular Formula | C15H10O5 |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 0 |
| Exact Mass | 270.05282342 g/mol |
| Monoisotopic Mass | 270.05282342 g/mol |
| Topological Polar Surface Area | 94.8 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 434 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Emodin /is/ a widely available over-the-counter herbal remedy.
George J et al; Toxicologist 72(S-1): 341 (2003)
/EXPL THER:/ ... Emodin and cassiamin B /were examined as cancer chemopreventive agents/ ... These compounds exhibited the remarkable anti-tumor promoting effect on two-stage carcinogenesis test of mouse skin tumors induced by 7,12-dimethylbenz[a]anthracene as an initiator and 12-O-tetradecanoylphorbol-13-acetate (TPA) as a promoter by both topical application. Furthermore, emodin exhibited potent inhibitory activity on two-stage carcinogenesis test of mouse skin tumors induced by nitric oxide donor, (+/-)-(E)-methyl-2-[(E)-hydroxyimino]-5-nitro-6-methoxy-3-hexeneamide as an initiator and TPA as a promoter.
PMID:12048158 Koyama J et al; Cancer Lett 182(2): 135-9 (2002)
/EXPL THER:/ ... Emodin ameliorates the undesirable effects of concentrated glucose on HPMC /human peritoneal mesothelial cells/ via suppression of PKC activation and CREB phosphorylation, and suggest that emodin may have a therapeutic potential in the prevention or treatment of glucose-induced structural and functional abnormalities in the peritoneal membrane.
PMID:12846747 Chan T et al; Kidney Int 64 (2): 519-533 (2003)
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
Cathartics
Agents that are used to stimulate evacuation of the bowels. (See all compounds classified as Cathartics.)
Absorption, excretion, tissue distribution and metabolism of the anthraquinone [14C]emodin was studied after a single oral administration (approx. 50 mg/kg) to rats. Urinary excretion amounted to 18(+/- 5)% dose in 24 hr and to 22(+/- 6)% in 72 hr. Metabolites found in pooled urine (0-72 hr) were mostly free anthraquinones (emodin and emodic acid, 16% dose); 3% was conjugated and 3% was non-extractable radioactivity. In 24 hr, 48 +/- 11% and in 120 hr, 68 +/- 8% dose was excreted in the faeces, mostly in the free anthraquinone form. In two cannulated rats biliary excretion reached a maximum at approx. 6 hr and amounted to 49% dose within 15 hr; 70% of biliary activity was in the form of conjugated emodin. The content of radioactivity in most organs decreased significantly between 3 and 5 days. In kidneys, however, the 14C activity was still equiv. to 4.33 ppm. emodin after five days. Mesenterium and fat tissue showed increasing 14C activity from 72 to 120 hr.
Bachmann M et al; Xenobiotica 11(3): 217-25 1981
... The metabolism of emodin (1,3,8-trihydroxy-6-methylanthraquinone) /was studied/... With rat liver microsomes, the formation of two emodin metabolites, omega-hydroxyemodin and 2-hydroxyemodin, was observed. The rates of formation of omega-hydroxyemodin were not different with microsomes from rats that had been pretreated with inducers for different cytochrome P450 enzymes. Thus, the formation of omega-hydroxyemodin seems to be catalyzed by several cytochrome P450 enzymes at low rates. The formation of 2-hydroxyemodin was increased in liver microsomes from 3-methylcholanthrene-pretreated rats and was inhibited by alpha-naphthoflavone, by an anti-rat cytochrome P450 1A1/2 antibody, and, to a lesser degree, by an anti-rat cytochrome P450 1A1 antibody. These data suggest the involvement of cytochrome P450 1A2 in the formation of this metabolite. However, other cytochrome P450 enzymes also seem to catalyze this reaction. The anthraquinone chrysophanol (1,8-dihydroxy-3-methylanthraquinone) is transformed, in a cytochrome P450-dependent oxidation, to aloe-emodin (1, 8-dihydroxy-3-hydroxymethylanthraquinone) as the major product formed.
PMID:9616189 Mueller S et al; Drug Metab Dispos. 26(6): 540-6 (1998)
The hepatic microsomes derived from various animal species transformed emodin (1,3,8-trihydroxy-6-methylanthraquinone), into an unidentified anthraquinone, along with 2-hydroxy-, 4-hydroxy- and 7-hydroxyemodins. ... This major metabolite /was identified/ as omega-hydroxy-emodin (1,3,8-trihydroxy-6-hydroxymethylanthraquinone). Among 7 animal species, the highest activity to produce this omega-hydroxyemodin was observed in the hepatic microsomes of guinea pig and rat, followed by mouse and rabbit. The microsomal activity to convert emodin into omega-hydroxyemodin was accelerated by the pretreatment of animals with phenobarbital, and inhibited by SKF 525A. The microsomal hydroxylation reactions of the methyl residue and the anthraquinoid nucleus of emodin were presumed to be catalyzed regiospecifically by multiple forms of cytochrome P-450.
PMID:3309636 Murakami H et al; Mutat Res 180 (2): 147-53 (1987)
... Emodin was biotransformed by the microsomal enzymes into at least 5 quinonoid metabolites, among which one pigment, identified as 2-hydroxyemodin (1,2,3,8-tetrahydroxy-6-methyl-anthraquinone), was proven to be a direct mutagen to the test strain, and the remaining 4 quinonoid metabolites were negative or far less active than this active principle.
PMID:6366529 Masuda T et al; Mutat Res 125(2): 135-44 (1984)
Emodin has known human metabolites that include Emodin 3-hydroxy-glucuronide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The anthraquinone mycotoxins emodin and skyrin were examined for the inhibitory effects on murine leukemia L1210 culture cells, oxidative phosphorylation of rat liver mitochondria, and Na+, K+-activated ATPase activity of rat brain microsomes to find the differences between their modes of toxic action. Skyrin exhibited a stronger inhibitory effect than emodin on the growth of L1210 culture cells. Emodin showed a stronger uncoupling effect than skyrin on mitochondrial respiration. Skyrin inhibited Na+, K+-activated ATPase activity of rat brain microsomes but emodin did not inhibit.
PMID:6320499 Kawai K et al; Toxicol Lett 20 (2): 155-60 (1984)
... Emodin induces apoptotic responses in the human hepatocellular carcinoma cell lines (HCC) Mahlavu, PLC / PRF / 5 and HepG2. The addition of emodin to these three cell lines led to inhibition of growth in a time- and dose-dependent manner. Emodin generated reactive oxygen species (ROS) in these cells which brought about a reduction of the intracellular mitochondrial transmembrane potential (Deltaym), followed by the activation of caspase-9 and caspase-3, leading to DNA fragmentation and apoptosis.
PMID:12716464 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5927105 Jing X et al; Jpn J Cancer Res 93 (8): 874-82 (2002)
Emodin inhibited the activity of TPK and CK2 and the degradation of I-kappaB.
PMID:12703988 Zhu F et al; Ai Zheng 22 (4): 358-362 (2003)
... Emodin-induced apoptosis of CH27 cells does not involve modulation of endogenous Bcl-X(L) protein expression, but appears to be associated with the increased expression of cellular Bak and Bax proteins.
PMID:11522592 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1572914 Lee HZ et al; Br J Pharmacol 134 (1): 11-20 (2001)
For more Mechanism of Action (Complete) data for EMODIN (9 total), please visit the HSDB record page.
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
63
PharmaCompass offers a list of Emodin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Emodin manufacturer or Emodin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Emodin manufacturer or Emodin supplier.
PharmaCompass also assists you with knowing the Emodin API Price utilized in the formulation of products. Emodin API Price is not always fixed or binding as the Emodin Price is obtained through a variety of data sources. The Emodin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Emodin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Emodin, including repackagers and relabelers. The FDA regulates Emodin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Emodin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Emodin supplier is an individual or a company that provides Emodin active pharmaceutical ingredient (API) or Emodin finished formulations upon request. The Emodin suppliers may include Emodin API manufacturers, exporters, distributors and traders.
Emodin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Emodin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Emodin GMP manufacturer or Emodin GMP API supplier for your needs.
A Emodin CoA (Certificate of Analysis) is a formal document that attests to Emodin's compliance with Emodin specifications and serves as a tool for batch-level quality control.
Emodin CoA mostly includes findings from lab analyses of a specific batch. For each Emodin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Emodin may be tested according to a variety of international standards, such as European Pharmacopoeia (Emodin EP), Emodin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Emodin USP).
